Transcription factor competition at the γ-globin promoters controls hemoglobin switching
- PMID: 33649594
- PMCID: PMC8038971
- DOI: 10.1038/s41588-021-00798-y
Transcription factor competition at the γ-globin promoters controls hemoglobin switching
Erratum in
-
Author Correction: Transcription factor competition at the γ-globin promoters controls hemoglobin switching.Nat Genet. 2021 Apr;53(4):586. doi: 10.1038/s41588-021-00834-x. Nat Genet. 2021. PMID: 33731942 Free PMC article. No abstract available.
Abstract
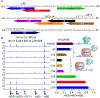
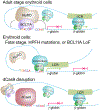
BCL11A, the major regulator of fetal hemoglobin (HbF, α2γ2) level, represses γ-globin expression through direct promoter binding in adult erythroid cells in a switch to adult hemoglobin (HbA, α2β2). To uncover how BCL11A initiates repression, we used CRISPR-Cas9, dCas9, dCas9-KRAB and dCas9-VP64 screens to dissect the γ-globin promoters and identified an activator element near the BCL11A-binding site. Using CUT&RUN and base editing, we demonstrate that a proximal CCAAT box is occupied by the activator NF-Y. BCL11A competes with NF-Y binding through steric hindrance to initiate repression. Occupancy of NF-Y is rapidly established following BCL11A depletion, and precedes γ-globin derepression and locus control region (LCR)-globin loop formation. Our findings reveal that the switch from fetal to adult globin gene expression within the >50-kb β-globin gene cluster is initiated by competition between a stage-selective repressor and a ubiquitous activating factor within a remarkably discrete region of the γ-globin promoters.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures







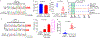

References
-
- Carter D, Chakalova L, Osborne CS, Dai Y & Fraser P Long-range chromatin regulatory interactions in vivo. Nat. Genet 32, 623–626 (2002). - PubMed
-
- Tolhuis B et al. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell 10, 1453–65 (2002). - PubMed
-
- Palstra RJ et al. The β-globin nuclear compartment in development and erythroid differentiation. Nature Genetics vol. 35 190–194 (2003). - PubMed
-
- Chada K, Magram J & Costantini F An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature 319, 685–689 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

