Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters
- PMID: 33649747
- PMCID: PMC7904445
- DOI: 10.1016/j.xcrm.2021.100218
Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters
Abstract
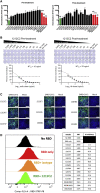
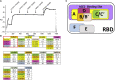
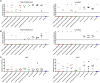
SARS-CoV-2 infection results in viral burden in the respiratory tract, enabling transmission and leading to substantial lung pathology. The 1212C2 fully human monoclonal antibody was derived from an IgM memory B cell of a COVID-19 patient, has high affinity for the Spike protein receptor binding domain, neutralizes SARS-CoV-2, and exhibits in vivo prophylactic and therapeutic activity in hamsters when delivered intraperitoneally, reducing upper and lower respiratory viral burden and lung pathology. Inhalation of nebulized 1212C2 at levels as low as 0.6 mg/kg, corresponding to 0.03 mg/kg lung-deposited dose, reduced the viral burden below the detection limit and mitigated lung pathology. The therapeutic efficacy of an exceedingly low dose of inhaled 1212C2 supports the rationale for local lung delivery for dose-sparing benefits, as compared to the conventional parenteral route of administration. These results suggest that the clinical development of 1212C2 formulated and delivered via inhalation for the treatment of SARS-CoV-2 infection should be considered.
Keywords: B cell; COVID-19; IgM; RBD; SARS-CoV-2; Spike; hamsters; inhalation; monoclonal antibody; neutralizing.
© 2021 The Author(s).
Conflict of interest statement
M.S.P., J.-G.P., A.D., F.S.O., M.B., S.S., N.B.E., P.A.G., M.R.W., L.M.-S., and J.J.K. are co-inventors on a patent that includes claims related to the hmAbs described. A.L., J.W., P.L., D.S., and V.L.T. are employees of Aridis Pharmaceuticals.
Figures



References
-
- Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., He Q., Wang Z., Liu Y., Liu L. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

