X-ray-Based Techniques to Study the Nano-Bio Interface
- PMID: 33650433
- PMCID: PMC7992135
- DOI: 10.1021/acsnano.0c09563
X-ray-Based Techniques to Study the Nano-Bio Interface
Abstract
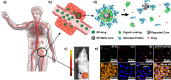
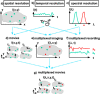
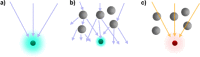
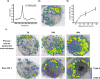
X-ray-based analytics are routinely applied in many fields, including physics, chemistry, materials science, and engineering. The full potential of such techniques in the life sciences and medicine, however, has not yet been fully exploited. We highlight current and upcoming advances in this direction. We describe different X-ray-based methodologies (including those performed at synchrotron light sources and X-ray free-electron lasers) and their potentials for application to investigate the nano-bio interface. The discussion is predominantly guided by asking how such methods could better help to understand and to improve nanoparticle-based drug delivery, though the concepts also apply to nano-bio interactions in general. We discuss current limitations and how they might be overcome, particularly for future use in vivo.
Keywords: X-ray techniques; degradation; delivery; imaging; nanoparticles; nano−bio interface; spectroscopy; synchrotron radiation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

