Safety and chronic lesion characterization of pulsed field ablation in a Porcine model
- PMID: 33650743
- PMCID: PMC8048690
- DOI: 10.1111/jce.14980
Safety and chronic lesion characterization of pulsed field ablation in a Porcine model
Abstract
Background: Pulsed field ablation (PFA) has been identified as an alternative to thermal-based ablation systems for treatment of atrial fibrillation patients. The objective of this Good Laboratory Practice (GLP) study was to characterize the chronic effects and safety of overlapping lesions created by a PFA system at intracardiac locations in a porcine model.
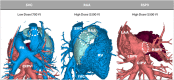
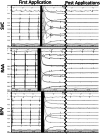
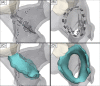
Methods: A circular catheter with nine gold electrodes was used for overlapping low- or high-dose PFA deliveries in the superior vena cava (SVC), right atrial appendage (RAA), and right superior pulmonary vein (RSPV) in six pigs. Electrical isolation was evaluated acutely and chronic lesions were assessed via necropsy and histopathology after 4-week survival. Acute and chronic safety data were recorded peri- and post-procedurally.
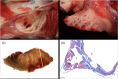
Results: No animal experienced ventricular arrhythmia during PFA delivery, and there was no evidence of periprocedural PFA-related adverse events. Lesions created in all anatomies resulted in electrical isolation postprocedure. Lesions were circumferential, contiguous, and transmural, with all converting into consistent lines of chronic replacement fibrosis, regardless of trabeculated or smooth endocardial surface structure. Ablations were non-thermally generated with only minimal post-delivery temperature rises recorded at the electrodes. There was no evidence of extracardiac damage, stenosis, aneurysms, endocardial disruption, or thrombus.
Conclusion: PFA deliveries to the SVC, RAA, and RSPV resulted in complete circumferential replacement fibrosis at 4-week postablation with an excellent chronic myocardial and collateral tissue safety profile. This GLP study evaluated the safety and efficacy of a dosage range in preparation for a clinical trial and characterized the non-thermal nature of PFA.
Keywords: atrial fibrillation; catheter ablation; electroporation; pulsed electric field modeling; pulsed field ablation.
© 2021 The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Conflict of interest statement
David Haines, Atul Verma, Damijan Miklavčič, and Bor Kos receive research and consultation funds from Medtronic, Inc. Nicole Kirchhof, Noah Barka, Lars Mattison, Matthew Martien, Birce Onal, Brian Howard, and Mark Stewart are employees of Medtronic, Inc.
Figures



Comment in
-
Novel energy source for ablating the pulmonary veins; Is pulsed field ablation the new ablation modality?J Cardiovasc Electrophysiol. 2021 Apr;32(4):970-972. doi: 10.1111/jce.14982. Epub 2021 Mar 10. J Cardiovasc Electrophysiol. 2021. PMID: 33650733 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

