Effect of the order of concurrent training combined with resistance and high-intensity interval exercise on mTOR signaling and glycolytic metabolism in mouse skeletal muscle
- PMID: 33650809
- PMCID: PMC7923557
- DOI: 10.14814/phy2.14770
Effect of the order of concurrent training combined with resistance and high-intensity interval exercise on mTOR signaling and glycolytic metabolism in mouse skeletal muscle
Abstract
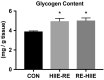
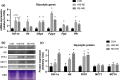
Athletes train to improve strength and endurance to demonstrate maximum performance during competitions. Training methods vary but most focus on strength, endurance, or both. Concurrent training is a combination of two different modes of training. In this study, we combined resistance exercise (RE) and high-intensity interval exercise (HIIE) to investigate the influence of the order of the concurrent training on signal molecules on hypertrophy and glycolysis in the skeletal muscle. The phosphorylation levels of mechanistic target of rapamycin (mTOR) signals, p70 S6 kinase (p70S6 K), ribosomal protein S6 (S6), and glycogen synthase kinase beta (GSK-3β) were significantly increased in the HIIE first group compared with the control group. The combined training course did not affect the glycogen content and expression levels of proteins concerning glycolytic and metabolic capacity, suggesting that a combination of HIIE and RE on the same day, with HIIE prior to RE, improves hypertrophy response and glycolysis enhancement.
Keywords: concurrent training; glycolysis; high-intensity interval exercise; mTOR signaling; resistance exercise.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
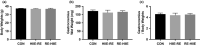


References
-
- Abe, T. , Kitaoka, Y. , Kikuchi, D. M. , Takeda, K. , Numata, O. , & Takemasa, T. (2015). High‐intensity interval training‐induced metabolic adaptation coupled with an increase in Hif‐1α and glycolytic protein expression. Journal of Applied Physiology, 1985(119), 1297–1302. - PubMed
-
- Ameln, H. , Gustafsson, T. , Sundberg, C. J. , Okamoto, K. , Jansson, E. , Poellinger, L. , & Makino, Y. (2005). Physiological activation of hypoxia inducible factor‐1 in human skeletal muscle. The FASEB Journal, 19, 1009–1011. - PubMed
-
- Biglari, S. , Afousi, A. G. , Mafi, F. , & Shabkhiz, F. (2020). High‐intensity interval training‐induced hypertrophy in gastrocnemius muscle via improved IGF‐I/Akt/FoxO and myostatin/Smad signaling pathways in rats. Physiology International, 107(2), 220–230. - PubMed
-
- Bishop, D. , Edge, J. , Davis, C. , & Goodman, C. (2004). Induced metabolic alkalosis affects muscle metabolism and repeated‐sprint ability. Medicine and Science in Sports and Exercise, 36, 807–813. - PubMed
-
- Bodine, S. C. , Stitt, T. N. , Gonzalez, M. , Kline, W. O. , Stover, G. L. , Bauerlein, R. , Zlotchenko, E. , Scrimgeour, A. , Lawrence, J. C. , Glass, D. J. , & Yancopoulos, G. D. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology, 3, 1014–1019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

