In vivo reconstitution finds multivalent RNA-RNA interactions as drivers of mesh-like condensates
- PMID: 33650968
- PMCID: PMC7968931
- DOI: 10.7554/eLife.64252
In vivo reconstitution finds multivalent RNA-RNA interactions as drivers of mesh-like condensates
Abstract
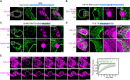
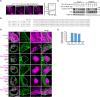
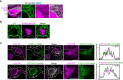
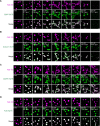
Liquid-like condensates have been thought to be sphere-like. Recently, various condensates with filamentous morphology have been observed in cells. One such condensate is the TIS granule network that shares a large surface area with the rough endoplasmic reticulum and is important for membrane protein trafficking. It has been unclear how condensates with mesh-like shapes but dynamic protein components are formed. In vitro and in vivo reconstitution experiments revealed that the minimal components are a multivalent RNA-binding protein that concentrates RNAs that are able to form extensive intermolecular mRNA-mRNA interactions. mRNAs with large unstructured regions have a high propensity to form a pervasive intermolecular interaction network that acts as condensate skeleton. The underlying RNA matrix prevents full fusion of spherical liquid-like condensates, thus driving the formation of irregularly shaped membraneless organelles. The resulting large surface area may promote interactions at the condensate surface and at the interface with other organelles.
Keywords: RNA biology; RNA multivalency; RNA-RNA interactions; biomolecular condensates; cell biology; condensate morphology; human; in vivo reconstitution.
© 2021, Ma et al.
Conflict of interest statement
WM, GZ, WX, CM No competing interests declared
Figures










Similar articles
-
Using quantitative reconstitution to investigate multicomponent condensates.RNA. 2022 Jan;28(1):27-35. doi: 10.1261/rna.079008.121. Epub 2021 Nov 12. RNA. 2022. PMID: 34772789 Free PMC article. Review.
-
RNA in formation and regulation of transcriptional condensates.RNA. 2022 Jan;28(1):52-57. doi: 10.1261/rna.078997.121. Epub 2021 Nov 12. RNA. 2022. PMID: 34772787 Free PMC article. Review.
-
Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies.Nat Commun. 2021 Feb 8;12(1):872. doi: 10.1038/s41467-021-21089-4. Nat Commun. 2021. PMID: 33558506 Free PMC article.
-
RNA-driven phase transitions in biomolecular condensates.Mol Cell. 2024 Oct 3;84(19):3692-3705. doi: 10.1016/j.molcel.2024.09.005. Mol Cell. 2024. PMID: 39366355 Review.
-
Splicing regulation through biomolecular condensates and membraneless organelles.Nat Rev Mol Cell Biol. 2024 Sep;25(9):683-700. doi: 10.1038/s41580-024-00739-7. Epub 2024 May 21. Nat Rev Mol Cell Biol. 2024. PMID: 38773325 Free PMC article. Review.
Cited by
-
The Role of Alternative Polyadenylation in the Regulation of Subcellular RNA Localization.Front Genet. 2022 Jan 14;12:818668. doi: 10.3389/fgene.2021.818668. eCollection 2021. Front Genet. 2022. PMID: 35096024 Free PMC article. Review.
-
Hitting the mark: Localization of mRNA and biomolecular condensates in health and disease.Wiley Interdiscip Rev RNA. 2023 Nov-Dec;14(6):e1807. doi: 10.1002/wrna.1807. Epub 2023 Jul 2. Wiley Interdiscip Rev RNA. 2023. PMID: 37393916 Free PMC article. Review.
-
Identification of Phase-Separation-Protein-Related Function Based on Gene Ontology by Using Machine Learning Methods.Life (Basel). 2023 May 31;13(6):1306. doi: 10.3390/life13061306. Life (Basel). 2023. PMID: 37374089 Free PMC article.
-
CIZ1 in Xist seeded assemblies at the inactive X chromosome.Front Cell Dev Biol. 2023 Dec 13;11:1296600. doi: 10.3389/fcell.2023.1296600. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38155839 Free PMC article. Review.
-
Poly(ADP-ribose) in Condensates: The PARtnership of Phase Separation and Site-Specific Interactions.Int J Mol Sci. 2022 Nov 15;23(22):14075. doi: 10.3390/ijms232214075. Int J Mol Sci. 2022. PMID: 36430551 Free PMC article. Review.
References
-
- Athanassiou Z, Dias RL, Moehle K, Dobson N, Varani G, Robinson JA. Structural mimicry of retroviral tat proteins by constrained beta-hairpin peptidomimetics: ligands with high affinity and selectivity for viral TAR RNA regulatory elements. Journal of the American Chemical Society. 2004;126:6906–6913. doi: 10.1021/ja0497680. - DOI - PubMed
-
- Aw JG, Shen Y, Wilm A, Sun M, Lim XN, Boon KL, Tapsin S, Chan YS, Tan CP, Sim AY, Zhang T, Susanto TT, Fu Z, Nagarajan N, Wan Y. In vivo mapping of eukaryotic RNA interactomes reveals principles of Higher-Order organization and regulation. Molecular Cell. 2016;62:603–617. doi: 10.1016/j.molcel.2016.04.028. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

