Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial
- PMID: 33651091
- PMCID: PMC7926287
- DOI: 10.1001/jama.2021.0987
Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial
Abstract
Importance: Blinatumomab is a CD3/CD19-directed bispecific T-cell engager molecule with efficacy in children with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Objective: To evaluate event-free survival in children with high-risk first-relapse B-ALL after a third consolidation course with blinatumomab vs consolidation chemotherapy before allogeneic hematopoietic stem cell transplant.
Design, setting, and participants: In this randomized phase 3 clinical trial, patients were enrolled November 2015 to July 2019 (data cutoff, July 17, 2019). Investigators at 47 centers in 13 countries enrolled children older than 28 days and younger than 18 years with high-risk first-relapse B-ALL in morphologic complete remission (M1 marrow, <5% blasts) or with M2 marrow (blasts ≥5% and <25%) at randomization.
Intervention: Patients were randomized to receive 1 cycle of blinatumomab (n = 54; 15 μg/m2/d for 4 weeks, continuous intravenous infusion) or chemotherapy (n = 54) for the third consolidation.
Main outcomes and measures: The primary end point was event-free survival (events: relapse, death, second malignancy, or failure to achieve complete remission). The key secondary efficacy end point was overall survival. Other secondary end points included minimal residual disease remission and incidence of adverse events.
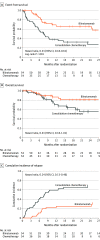
Results: A total of 108 patients were randomized (median age, 5.0 years [interquartile range {IQR}, 4.0-10.5]; 51.9% girls; 97.2% M1 marrow) and all patients were included in the analysis. Enrollment was terminated early for benefit of blinatumomab in accordance with a prespecified stopping rule. After a median of 22.4 months of follow-up (IQR, 8.1-34.2), the incidence of events in the blinatumomab vs consolidation chemotherapy groups was 31% vs 57% (log-rank P < .001; hazard ratio [HR], 0.33 [95% CI, 0.18-0.61]). Deaths occurred in 8 patients (14.8%) in the blinatumomab group and 16 (29.6%) in the consolidation chemotherapy group. The overall survival HR was 0.43 (95% CI, 0.18-1.01). Minimal residual disease remission was observed in more patients in the blinatumomab vs consolidation chemotherapy group (90% [44/49] vs 54% [26/48]; difference, 35.6% [95% CI, 15.6%-52.5%]). No fatal adverse events were reported. In the blinatumomab vs consolidation chemotherapy group, the incidence of serious adverse events was 24.1% vs 43.1%, respectively, and the incidence of adverse events greater than or equal to grade 3 was 57.4% vs 82.4%. Adverse events leading to treatment discontinuation were reported in 2 patients in the blinatumomab group.
Conclusions and relevance: Among children with high-risk first-relapse B-ALL, treatment with 1 cycle of blinatumomab compared with standard intensive multidrug chemotherapy before allogeneic hematopoietic stem cell transplant resulted in an improved event-free survival at a median of 22.4 months of follow-up.
Trial registration: ClinicalTrials.gov Identifier: NCT02393859.
Conflict of interest statement
Figures

Comment in
-
Blinatumomab for Treatment of Children With High-risk Relapsed B-Cell Acute Lymphoblastic Leukemia.JAMA. 2021 Mar 2;325(9):830-832. doi: 10.1001/jama.2021.1395. JAMA. 2021. PMID: 33651075 No abstract available.
-
Blinatumomab vs Chemotherapy Among Children With Relapsed Acute Lymphoblastic Leukemia.JAMA. 2021 Jul 27;326(4):359. doi: 10.1001/jama.2021.8151. JAMA. 2021. PMID: 34313694 No abstract available.
-
Interim Analyses During Group Sequential Clinical Trials.JAMA. 2021 Oct 19;326(15):1524-1525. doi: 10.1001/jama.2021.10174. JAMA. 2021. PMID: 34596661 No abstract available.

