A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells
- PMID: 33651100
- PMCID: PMC7948265
- DOI: 10.1182/bloodadvances.2020002736
A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells
Abstract
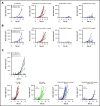
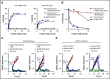
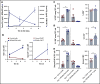
CD3-engaging bispecific antibodies (bsAbs) and chimeric antigen receptor (CAR) T cells are potent therapeutic approaches for redirecting patient T cells to recognize and kill tumors. Here we describe a fully human bsAb (REGN5458) that binds to B-cell maturation antigen (BCMA) and CD3, and compare its antitumor activities vs those of anti-BCMA CAR T cells to identify differences in efficacy and mechanism of action. In vitro, BCMAxCD3 bsAb efficiently induced polyclonal T-cell killing of primary human plasma cells and multiple myeloma (MM) cell lines expressing a range of BCMA cell surface densities. In vivo, BCMAxCD3 bsAb suppressed the growth of human MM tumors in murine xenogeneic models and showed potent combinatorial efficacy with programmed cell death protein 1 blockade. BCMAxCD3 bsAb administration to cynomolgus monkeys was well tolerated, resulting in the depletion of BCMA+ cells and mild inflammatory responses characterized by transient increases in C-reactive protein and serum cytokines. The antitumor efficacy of BCMAxCD3 bsAb was compared with BCMA-specific CAR T cells containing a BCMA-binding single-chain variable fragment derived from REGN5458. Both BCMAxCD3 bsAb and anti-BCMA CAR T cells showed similar targeted cytotoxicity of MM cell lines and primary MM cells in vitro. In head-to-head in vivo studies, BCMAxCD3 bsAb rapidly cleared established systemic MM tumors, whereas CAR T cells cleared tumors with slower kinetics. Thus, using the same BCMA-binding domain, these results suggest that BCMAxCD3 bsAb rapidly exerts its therapeutic effects by engaging T cells already in place at the tumor site, whereas anti-BCMA CAR T cells require time to traffic to the tumor site, activate, and numerically expand before exerting antitumor effects.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: All authors are employees and equity-shareholders of Regeneron Pharmaceuticals.
Figures




References
-
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. - PubMed
-
- Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443-2448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

