The Roles of Serotonin in Neuropsychiatric Disorders
- PMID: 33651238
- PMCID: PMC11421740
- DOI: 10.1007/s10571-021-01064-9
The Roles of Serotonin in Neuropsychiatric Disorders
Abstract
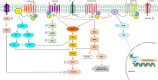
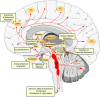
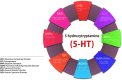
The serotonergic system extends throughout the central nervous system (CNS) and the gastrointestinal (GI) tract. In the CNS, serotonin (5-HT, 5-hydroxytryptamine) modulates a broad spectrum of functions, including mood, cognition, anxiety, learning, memory, reward processing, and sleep. These processes are mediated through 5-HT binding to 5-HT receptors (5-HTRs), are classified into seven distinct groups. Deficits in the serotonergic system can result in various pathological conditions, particularly depression, schizophrenia, mood disorders, and autism. In this review, we outlined the complexity of serotonergic modulation of physiologic and pathologic processes. Moreover, we provided experimental and clinical evidence of 5-HT's involvement in neuropsychiatric disorders and discussed the molecular mechanisms that underlie these illnesses and contribute to the new therapies.
Keywords: 5-HT; Mood Disorders; Nervous System Diseases; SSRI; Serotonin pathway.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC part of Springer Nature.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this paper.
Figures

References
-
- Abela AR, Browne CJ, Sargin D, Prevot TD, Ji XD, Li Z, Lambe EK, Fletcher PJ (2020) Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology 15(168):107985. 10.1016/j.neuropharm.2020.107985 - PubMed
-
- Adayev T, Ranasinghe B, Banerjee P (2005) Transmembrane signaling in the brain by serotonin, a key regulator of physiology and emotion. Biosci Rep 25:363–385. 10.1007/s10540-005-2896-3 - PubMed
-
- Akhondzadeh S, Mohammadi N, Noroozian M, Karamghadiri N, Ghoreishi A, Jamshidi AH, Forghani S (2009) Added ondansetron for stable schizophrenia: a double blind, placebo controlled trial. Schizophrenia research Schizophr Res 107(2–3):206–212. 10.1016/j.schres.2008.08.004 - PubMed
-
- Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, Michel TM (2017) Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry 45:161–166. 10.1016/j.eurpsy.2017.06.001 - PubMed
-
- Andreetta F, Carboni L, Grafton G, Jeggo R, Whyment AD, Van Den Top M, Hoyer D, Spanswick D, Barnes NM (2016) Hippocampal 5-HT7 receptors signal phosphorylation of the GluA1 subunit to facilitate AMPA receptor mediated-neurotransmission in vitro and in vivo. Pharmacol 173:1438–1451. 10.1111/bph.13432 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

