Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis-associated Pulmonary Arterial Hypertension: A Multicenter, Double-Blind, Randomized, Placebo-controlled Trial
- PMID: 33651671
- PMCID: PMC8650794
- DOI: 10.1164/rccm.202009-3481OC
Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis-associated Pulmonary Arterial Hypertension: A Multicenter, Double-Blind, Randomized, Placebo-controlled Trial
Abstract
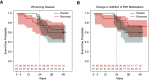
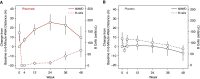
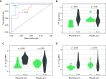
Rationale: Systemic sclerosis (SSc)-pulmonary arterial hypertension (PAH) is one of the most prevalent and deadly forms of PAH. B cells may contribute to SSc pathogenesis. Objectives: We investigated the safety and efficacy of B-cell depletion for SSc-PAH. Methods: In an NIH-sponsored, multicenter, double-blinded, randomized, placebo-controlled, proof-of-concept trial, 57 patients with SSc-PAH on stable-dose standard medical therapy received two infusions of 1,000 mg rituximab or placebo administered 2 weeks apart. The primary outcome measure was the change in 6-minute-walk distance (6MWD) at 24 weeks. Secondary endpoints included safety and invasive hemodynamics. We applied a machine learning approach to predict drug responsiveness. Measurements and Main Results: We randomized 57 subjects from 2010 to 2018. In the primary analysis, using data through Week 24, the adjusted mean change in 6MWD at 24 weeks favored the treatment arm but did not reach statistical significance (23.6 ± 11.1 m vs. 0.5 ± 9.7 m; P = 0.12). Although a negative study, when data through Week 48 were also considered, the estimated change in 6MWD at Week 24 was 25.5 ± 8.8 m for rituximab and 0.4 ± 7.4 m for placebo (P = 0.03). Rituximab treatment appeared to be safe and well tolerated. Low levels of RF (rheumatoid factor), IL-12, and IL-17 were sensitive and specific as favorable predictors of a rituximab response as measured by an improved 6MWD (receiver operating characteristic area under the curve, 0.88-0.95). Conclusions: B-cell depletion therapy is a potentially effective and safe adjuvant treatment for SSc-PAH. Future studies in these patients can confirm whether the identified biomarkers predict rituximab responsiveness. Clinical trial registered with www.clinicaltrails.gov (NCT01086540).
Keywords: pulmonary hypertension; systemic sclerosis; treatment.
Figures


Comment in
-
A Phase-2 NIH-sponsored Randomized Clinical Trial of Rituximab in Scleroderma-associated Pulmonary Arterial Hypertension Did Not Reach Significance for Its Endpoints: End of Story? Not So Fast!Am J Respir Crit Care Med. 2021 Jul 15;204(2):123-125. doi: 10.1164/rccm.202103-0612ED. Am J Respir Crit Care Med. 2021. PMID: 33856964 Free PMC article. No abstract available.
-
Reply to Andréasson et al.: Multiple Manifestations of Systemic Sclerosis Affect Walk Distance.Am J Respir Crit Care Med. 2021 Aug 1;204(3):377-378. doi: 10.1164/rccm.202104-1023LE. Am J Respir Crit Care Med. 2021. PMID: 34107229 Free PMC article. No abstract available.
-
Multiple Manifestations of Systemic Sclerosis Affect Walk Distance.Am J Respir Crit Care Med. 2021 Aug 1;204(3):359. doi: 10.1164/rccm.202104-0938LE. Am J Respir Crit Care Med. 2021. PMID: 34107236 Free PMC article. No abstract available.
References
-
- Steen VD, Medsger TA., Jr Epidemiology and natural history of systemic sclerosis. Rheum Dis Clin North Am . 1990;16:1–10. - PubMed
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med . 2009;360:1989–2003. - PubMed
-
- Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004;50:1918–1927. - PubMed
-
- Yoshizaki A. B lymphocytes in systemic sclerosis: abnormalities and therapeutic targets. J Dermatol . 2016;43:39–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL095686/HL/NHLBI NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- R01 HL141105/HL/NHLBI NIH HHS/United States
- P01 HL108797/HL/NHLBI NIH HHS/United States
- U01 HL107393/HL/NHLBI NIH HHS/United States
- U19 AI046374/AI/NIAID NIH HHS/United States
- K23 HL151892/HL/NHLBI NIH HHS/United States
- R01 HL138473/HL/NHLBI NIH HHS/United States
- UM1 AI110498/AI/NIAID NIH HHS/United States
- U19 AI056363/AI/NIAID NIH HHS/United States
- R01 HL082662/HL/NHLBI NIH HHS/United States
- U19 AI110491/AI/NIAID NIH HHS/United States
- P01 HL014985/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

