DUOX2 variants associate with preclinical disturbances in microbiota-immune homeostasis and increased inflammatory bowel disease risk
- PMID: 33651715
- PMCID: PMC8087203
- DOI: 10.1172/JCI141676
DUOX2 variants associate with preclinical disturbances in microbiota-immune homeostasis and increased inflammatory bowel disease risk
Abstract
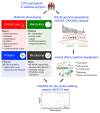
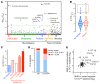
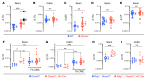
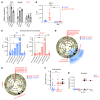
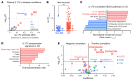
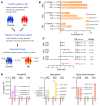
A primordial gut-epithelial innate defense response is the release of hydrogen peroxide by dual NADPH oxidase (DUOX). In inflammatory bowel disease (IBD), a condition characterized by an imbalanced gut microbiota-immune homeostasis, DUOX2 isoenzyme is the highest induced gene. Performing multiomic analyses using 2872 human participants of a wellness program, we detected a substantial burden of rare protein-altering DUOX2 gene variants of unknown physiologic significance. We identified a significant association between these rare loss-of-function variants and increased plasma levels of interleukin-17C, which is induced also in mucosal biopsies of patients with IBD. DUOX2-deficient mice replicated increased IL-17C induction in the intestine, with outlier high Il17c expression linked to the mucosal expansion of specific Proteobacteria pathobionts. Integrated microbiota/host gene expression analyses in patients with IBD corroborated IL-17C as a marker for epithelial activation by gram-negative bacteria. Finally, the impact of DUOX2 variants on IL-17C induction provided a rationale for variant stratification in case control studies that substantiated DUOX2 as an IBD risk gene. Thus, our study identifies an association of deleterious DUOX2 variants with a preclinical hallmark of disturbed microbiota-immune homeostasis that appears to precede the manifestation of IBD.
Keywords: Gastroenterology; Genetic variation; Inflammatory bowel disease; Innate immunity.
Conflict of interest statement
Figures

Comment in
-
Decoding the matrix: multiomics reveals host-microbe biomarker for inflammatory bowel disease.J Clin Invest. 2021 May 3;131(9):e148902. doi: 10.1172/JCI148902. J Clin Invest. 2021. PMID: 33938448 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

