B cell-activating factor modulates the factor VIII immune response in hemophilia A
- PMID: 33651716
- PMCID: PMC8262462
- DOI: 10.1172/JCI142906
B cell-activating factor modulates the factor VIII immune response in hemophilia A
Abstract
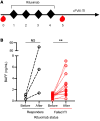
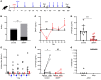
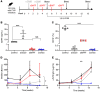
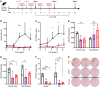
Inhibitors of factor VIII (FVIII) remain the most challenging complication of FVIII protein replacement therapy in hemophilia A (HA). Understanding the mechanisms that guide FVIII-specific B cell development could help identify therapeutic targets. The B cell-activating factor (BAFF) cytokine family is a key regulator of B cell differentiation in normal homeostasis and immune disorders. Thus, we used patient samples and mouse models to investigate the potential role of BAFF in modulating FVIII inhibitors. BAFF levels were elevated in pediatric and adult HA inhibitor patients and decreased to levels similar to those of noninhibitor controls after successful immune tolerance induction (ITI). Moreover, elevations in BAFF levels were seen in patients who failed to achieve FVIII tolerance with anti-CD20 antibody-mediated B cell depletion. In naive HA mice, prophylactic anti-BAFF antibody therapy prior to FVIII immunization prevented inhibitor formation and this tolerance was maintained despite FVIII exposure after immune reconstitution. In preimmunized HA mice, combination therapy with anti-CD20 and anti-BAFF antibodies dramatically reduced FVIII inhibitors via inhibition of FVIII-specific plasma cells. Our data suggest that BAFF may regulate the generation and maintenance of FVIII inhibitors and/or anti-FVIII B cells. Finally, anti-CD20/anti-BAFF combination therapy may be clinically useful for ITI.
Keywords: Coagulation; Cytokines; Hematology.
Conflict of interest statement
Figures


References
-
- Lossing TS, et al. Detection of factor VIII inhibitors with the partial thromboplastin time. Blood. 1977;49(5):793–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

