Variable host responses mediate host preference in marine flatworm-snail symbioses
- PMID: 33651807
- PMCID: PMC7924752
- DOI: 10.1371/journal.pone.0247551
Variable host responses mediate host preference in marine flatworm-snail symbioses
Abstract
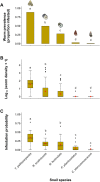
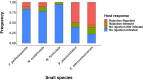
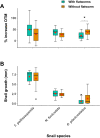
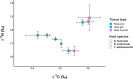
Host preference of symbionts evolves from fitness trade-offs. However, it is often unclear how interspecific variations in host response traits influence this evolutionary process. Using the association between the polyclad flatworm Paraprostatum echinolittorinae and its intertidal snail hosts on the Pacific Coast of Panama, we assessed how a symbiont's host preference is associated with varying host defenses and post-infestation performances. We first characterized the prevalence and intensity of worm infestation in five snail hosts (Tegula pellisserpentis, Nerita scabricosta, N. funiculata, Planaxis planicostatus, and Cerithium stercusmuscarum). We then used manipulative experiments to test flatworm's host choice, hosts' behavioral rejection of flatworms, and hosts' growth and survival following the infestation. In the field, flatworms were orders of magnitude more prevalent and dense in T. pellisserpentis, N. scabricosta, N. funiculata than P. planicostatus and C. stercusmuscarum, although the three former hosts were not necessarily more abundant. The results from our laboratory host selection trials mirrored these patterns; flatworms were 3 to 14 times more likely to choose T. pellisserpentis, N. scabricosta, N. funiculata over P. planicostatus and C. stercusmuscarum. The less preferred hosts frequently rejected flatworms via mantle contractions and foot withdrawals, which reduced the infestation rate by 39%-67%. These behaviors were less frequent or absent in the preferred hosts. Flatworm infestation variably influenced host performances in the field, negligibly affecting the growth and survival of T. pellisserpentis and N. funiculata but reducing the growth of P. planicostatus. Flatworms thus preferred less defended hosts that can also support higher worm densities without being harmed. Stable isotope analysis further revealed that flatworms are unlikely to feed on snail tissues and may live as a commensal in their preferred hosts. Our study demonstrates that host response traits can modulate a symbiont's host choice and calls for more explicit considerations of host response variability in host preference research.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- De Bary A. Die Erscheinung der Symbiose: Verlag von Karl J. Trübner Strassburg; 1879.
-
- Ocampo EH, Nuñez JD, Cledón M, Baeza JA. Host‐specific reproductive benefits, host selection behavior and host use pattern of the pinnotherid crab Calyptraeotheres garthi. J Exp Mar Biol Ecol. 2012;429:36–46.
-
- Poore AG, Steinberg PD. Preference–performance relationships and effects of host plant choice in an herbivorous marine amphipod. Ecol Monogr. 1999;69(4):443–464.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

