Excessive R-loops trigger an inflammatory cascade leading to increased HSPC production
- PMID: 33651979
- PMCID: PMC8258699
- DOI: 10.1016/j.devcel.2021.02.006
Excessive R-loops trigger an inflammatory cascade leading to increased HSPC production
Abstract
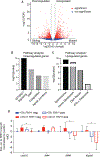
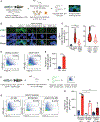
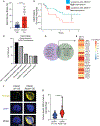
Hematopoietic stem and progenitor cells (HSPCs) arise during embryonic development and are essential for sustaining the blood and immune systems throughout life. Tight regulation of HSPC numbers is critical for hematopoietic homeostasis. Here, we identified DEAD-box helicase 41 (Ddx41) as a gatekeeper of HSPC production. Using zebrafish ddx41 mutants, we unveiled a critical role for this helicase in regulating HSPC production at the endothelial-to-hematopoietic transition. We determined that Ddx41 suppresses the accumulation of R-loops, nucleic acid structures consisting of RNA:DNA hybrids and ssDNAs whose equilibrium is essential for cellular fitness. Excess R-loop levels in ddx41 mutants triggered the cGAS-STING inflammatory pathway leading to increased numbers of hemogenic endothelium and HSPCs. Elevated R-loop accumulation and inflammatory signaling were observed in human cells with decreased DDX41, suggesting possible conservation of mechanism. These findings delineate that precise regulation of R-loop levels during development is critical for limiting cGAS-STING activity and HSPC numbers.
Keywords: Ddx41; R-loops; hematopoietic stem and progenitor cell; inflammatory signaling; zebrafish.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
Ddx41 loss R-loops in cGAS to fuel inflammatory HSPC production.Dev Cell. 2021 Mar 8;56(5):571-572. doi: 10.1016/j.devcel.2021.02.014. Dev Cell. 2021. PMID: 33689767
References
-
- Boguslawski SJ, Smith DE, Michalak MA, Mickelson KE, Yehle CO, Patterson WL, and Carrico RJ (1986). Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J. Immunol. Methods 89, 123–130. - PubMed
-
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, and Robin C (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

