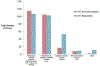Adherence rates during a randomized controlled trial evaluating the use of blinded acetaminophen and ibuprofen in children with asthma
- PMID: 33652129
- PMCID: PMC8180511
- DOI: 10.1016/j.cct.2021.106334
Adherence rates during a randomized controlled trial evaluating the use of blinded acetaminophen and ibuprofen in children with asthma
Abstract
Background/aims: When conducting clinical trials comparing over-the-counter (OTC) medications, the wide availability of these treatments are a potential challenge to maintaining study integrity. We seek to describe adherence to a study protocol involving widely available OTC medications.
Methods: To prospectively evaluate associations between acetaminophen use and asthma in 300 children aged 1-5 years, we conducted a double blind, randomized, controlled trial where parents administered blinded forms of either acetaminophen or ibuprofen as needed to their children over a 48 week period. Written and verbal instructions encouraged the exclusive use of the blinded study medication and discouraged OTC use. Adherence was determined by evaluating the frequency of use of per-protocol blinded study medication compared to off-protocol use of OTC medications.
Results: 4195 doses of acetaminophen or ibuprofen were received by children during the study which included 3664 doses (87.3%) of blinded study medication adhering to the protocol and 531 doses (12.7%) of OTC products deviating from the protocol with better adherence among those randomized to ibuprofen as compared to acetaminophen (89.5% vs. 85.5% of doses, p < 0.01). Individually, 227 participants (75.7%) remained fully adherent by not receiving any OTC medications. Pre-study preference for either acetaminophen or ibuprofen by the participants' families was not associated with differential rates of adherence to the blinded medication.
Conclusion: This parallel study demonstrated greater than 85% of acetaminophen or ibuprofen doses were blinded study medications adhering to the protocol while less than 15% were OTC deviations from the protocol. This successfully implemented study design provides a template to comparatively evaluate these and other OTC medications.
Keywords: Acetaminophen; Asthma; Children; Ibuprofen; Randomized controlled trial.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Nichol AD, Bailey M, Cooper DJ, Polar, Investigators EPO. Challenging issues in randomised controlled trials. Injury 2010;41 Suppl 1:S20–3. - PubMed
-
- Lesko SM, Louik C, Vezina RM, Mitchell AA. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics 2002;109:E20. - PubMed
-
- Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme. Lancet 2008;372:1039–48. - PubMed
-
- Beasley RW, Clayton TO, Crane J, et al. Acetaminophen use and risk of asthma, rhinoconjunctivitis, and eczema in adolescents: International Study of Asthma and Allergies in Childhood Phase Three. Am J Respir Crit Care Med 2011;183:171–8. - PubMed
-
- Davey G, Berhane Y, Duncan P, Aref-Adib G, Britton J, Venn A. Use of acetaminophen and the risk of self-reported allergic symptoms and skin sensitization in Butajira, Ethiopia. The Journal of allergy and clinical immunology 2005;116:863–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HL098107/HL/NHLBI NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
- K23 AI104780/AI/NIAID NIH HHS/United States
- U10 HL098096/HL/NHLBI NIH HHS/United States
- U10 HL098102/HL/NHLBI NIH HHS/United States
- U10 HL098177/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U10 HL098098/HL/NHLBI NIH HHS/United States
- K24 AI106822/AI/NIAID NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- U01 AI126614/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- U10 HL098090/HL/NHLBI NIH HHS/United States
- U10 HL098112/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical