Multidomain drug delivery systems of β-casein micelles for the local oral co-administration of antiretroviral combinations
- PMID: 33652169
- PMCID: PMC9749415
- DOI: 10.1016/j.jcis.2020.12.021
Multidomain drug delivery systems of β-casein micelles for the local oral co-administration of antiretroviral combinations
Abstract
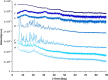
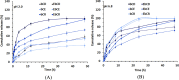
The antiretroviral (ARV) cocktailrevolved the treatment of the human immunodeficiency virus (HIV) infection. Drug combinations have been also tested to treat other infectious diseases, including the recentcoronavirus disease 2019 (COVID-19) outbreak. To simplify administration fixed-dose combinationshave been introduced, however, oral anti-HIV therapy still struggles with low oral bioavailability of many ARVs.This work investigated the co-encapsulation of two clinically relevant ARV combinations,tipranavir (TPV):efavirenz (EFV) anddarunavir (DRV):efavirenz (EFV):ritonavir (RTV),within the core of β-casein (bCN) micelles. Encapsulation efficiency in both systems was ~100%. Cryo-transmission electron microscopy and dynamic light scattering of the ARV-loaded colloidaldispersions indicatefull preservation of the spherical morphology, and x-ray diffraction confirm that the encapsulated drugs are amorphous. To prolong the physicochemical stabilitythe formulations were freeze-driedwithout cryo/lyoprotectant, and successfully redispersed, with minor changes in morphology.Then, theARV-loaded micelles were encapsulated within microparticles of Eudragit® L100, which prevented enzymatic degradation and minimized drug release under gastric-like pH conditionsin vitro. At intestinal pH, the coating polymer dissolved and released the nanocarriers and content. Overall, our results confirm the promise of this flexible and modular technology platform for oral delivery of fixed dose combinations.
Keywords: Antiretrovirals; Colloidal dispersion; Combination therapy; Darunavir, efavirenz and ritonavir; Nanoparticle-in-microparticle delivery system (NiMDS); cryo-TEM; β-casein micelles (bCN).
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Nanoparticle-in-microparticle oral drug delivery system of a clinically relevant darunavir/ritonavir antiretroviral combination.Acta Biomater. 2018 Jul 1;74:344-359. doi: 10.1016/j.actbio.2018.04.045. Epub 2018 May 1. Acta Biomater. 2018. PMID: 29723705
-
Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability [corrected].Nanomedicine (Lond). 2010 Jan;5(1):11-23. doi: 10.2217/nnm.09.90. Nanomedicine (Lond). 2010. PMID: 20025460
-
Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micelles.J Control Release. 2012 Jun 10;160(2):164-71. doi: 10.1016/j.jconrel.2012.01.004. Epub 2012 Jan 14. J Control Release. 2012. PMID: 22266050
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Exploring the use of novel drug delivery systems for antiretroviral drugs.Eur J Pharm Biopharm. 2008 Nov;70(3):697-710. doi: 10.1016/j.ejpb.2008.06.020. Epub 2008 Jul 3. Eur J Pharm Biopharm. 2008. PMID: 18655830 Review.
Cited by
-
Recent Progress in Proteins-Based Micelles as Drug Delivery Carriers.Polymers (Basel). 2023 Feb 8;15(4):836. doi: 10.3390/polym15040836. Polymers (Basel). 2023. PMID: 36850121 Free PMC article. Review.
-
Challenges and opportunities in delivering oral peptides and proteins.Expert Opin Drug Deliv. 2023 Jul-Dec;20(10):1349-1369. doi: 10.1080/17425247.2023.2237408. Epub 2023 Jul 17. Expert Opin Drug Deliv. 2023. PMID: 37450427 Free PMC article. Review.
-
Casein Micelles as an Emerging Delivery System for Bioactive Food Components.Foods. 2021 Aug 23;10(8):1965. doi: 10.3390/foods10081965. Foods. 2021. PMID: 34441743 Free PMC article. Review.
-
Nano drug-delivery systems for management of AIDS: liposomes, dendrimers, gold and silver nanoparticles.Nanomedicine (Lond). 2023 Feb;18(3):279-302. doi: 10.2217/nnm-2022-0248. Epub 2023 Apr 26. Nanomedicine (Lond). 2023. PMID: 37125616 Free PMC article. Review.
References
-
- The global HIV/AIDS epidemic. Available from: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics, 2019 (accessed on 3 May 2020).
-
- Hobson J.J., Al-khouja A., Curley P., Meyers D., Flexner C., Siccardi M., Owen A., Meyers C.F., Rannard S.P. Semi-solid prodrug nanoparticles for long-acting delivery of water-soluble antiretroviral drugs within combination HIV therapies. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-09354-z. - DOI - PMC - PubMed
-
- Cohen J. Can an anti-HIV combination or other existing drugs outwit the new coronavirus? Science. 2020 doi: 10.1126/science:abb0659. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

