Coronavirus and its terrifying inning around the globe: The pharmaceutical cares at the main frontline
- PMID: 33652275
- PMCID: PMC7884917
- DOI: 10.1016/j.chemosphere.2021.129968
Coronavirus and its terrifying inning around the globe: The pharmaceutical cares at the main frontline
Abstract
A novel coronavirus (2019-nCoV) is an acute life-threatening disease, emerged in China, which imposed a potentially immense toll in terms of public health emergency due to high infection rate and has a devastating economic impact that attracts the world's attention. After that, on January 30, 2020, it was officially declared as a global pandemic by World Health Organization (WHO). The International Committee on Taxonomy of Viruses (ICTV) recognized it as a Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and the disease named Coronavirus Disease-19 (COVID-19). Several studies have been ameliorated the active role of COVID-19 transmission, etiology, pathogenicity, and mortality rate as serious impact on human life. The symptoms of this disease may include fever, fatigue, cough and some peoples are severely prone to gastrointestinal infection. The elderly and seriously affected peoples are likely concerned with serious outcomes. In this review, we mainly aimed to provide a benchmark summary of the silent characteristics and findings of some candidates for antiviral drugs and immunotherapies such as plasma therapy, cytokine therapy, antibodies, intravenous immunoglobulin, and pharmaceutical health concerns that are related to this disease.
Keywords: COVID-19; Clinical characteristics; Diagnosis; Origin; Pharmaceutical services; SARS-CoV-2; Transmission.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
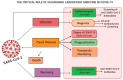

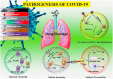
References
-
- Alvi M.M., Sivasankaran S., Singh M. Pharmacological and non-pharmacological efforts at prevention, mitigation, and treatment for COVID-19. J. Drug Target. 2020:1–13. - PubMed
-
- Amsden G. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 2005;55:10–21. - PubMed
-
- Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:1–13. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

