The Interplay of Cholesterol and Ligand Binding in h TSPO from Classical Molecular Dynamics Simulations
- PMID: 33652554
- PMCID: PMC7956637
- DOI: 10.3390/molecules26051250
The Interplay of Cholesterol and Ligand Binding in h TSPO from Classical Molecular Dynamics Simulations
Abstract
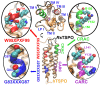
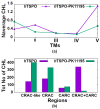
The translocator protein (TSPO) is a 18kDa transmembrane protein, ubiquitously present in human mitochondria. It is overexpressed in tumor cells and at the sites of neuroinflammation, thus representing an important biomarker, as well as a promising drug target. In mammalian TSPO, there are cholesterol-binding motifs, as well as a binding cavity able to accommodate different chemical compounds. Given the lack of structural information for the human protein, we built a model of human (h) TSPO in the apo state and in complex with PK11195, a molecule routinely used in positron emission tomography (PET) for imaging of neuroinflammatory sites. To better understand the interactions of PK11195 and cholesterol with this pharmacologically relevant protein, we ran molecular dynamics simulations of the apo and holo proteins embedded in a model membrane. We found that: (i) PK11195 stabilizes hTSPO structural fold; (ii) PK11195 might enter in the binding site through transmembrane helices I and II of hTSPO; (iii) PK11195 reduces the frequency of cholesterol binding to the lower, N-terminal part of hTSPO in the inner membrane leaflet, while this impact is less pronounced for the upper, C-terminal part in the outer membrane leaflet, where the ligand binding site is located; (iv) very interestingly, cholesterol most frequently binds simultaneously to the so-called CRAC and CARC regions in TM V in the free form (residues L150-X-Y152-X(3)-R156 and R135-X(2)-Y138-X(2)-L141, respectively). However, when the protein is in complex with PK11195, cholesterol binds equally frequently to the CRAC-resembling motif that we observed in TM I (residues L17-X(2)-F20-X(3)-R24) and to CRAC in TM V. We expect that the CRAC-like motif in TM I will be of interest in future experimental investigations. Thus, our MD simulations provide insight into the structural features of hTSPO and the previously unknown interplay between PK11195 and cholesterol interactions with this pharmacologically relevant protein.
Keywords: PK11195; cholesterol; hTSPO; homology modeling; molecular dynamics (MD) simulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Fan J., Lindemann P., GJ Feuilloley M., Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr. Mol. Med. 2012;12:369–386. - PubMed
-
- Yasin N., Veenman L., Singh S., Azrad M., Bode J., Vainshtein A., Caballero B., Marek I., Gavish M. Classical and novel TSPO ligands for the mitochondrial TSPO can modulate nuclear gene expression: Implications for mitochondrial retrograde signaling. Int. J. Mol. Sci. 2017;18:786. doi: 10.3390/ijms18040786. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

