Preclinical Evaluation of Artesunate as an Antineoplastic Agent in Ovarian Cancer Treatment
- PMID: 33652561
- PMCID: PMC7996621
- DOI: 10.3390/diagnostics11030395
Preclinical Evaluation of Artesunate as an Antineoplastic Agent in Ovarian Cancer Treatment
Abstract
Background: Ovarian cancer is the deadliest gynecologic malignancy despite current first-line treatment with a platinum and taxane doublet. Artesunate has broad antineoplastic properties but has not been investigated in combination with carboplatin and paclitaxel for ovarian cancer treatment.
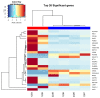
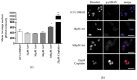
Methods: Standard cell culture technique with commercially available ovarian cancer cell lines were utilized in cell viability, DNA damage, and cell cycle progression assays to qualify and quantify artesunate treatment effects. Additionally, the sequence of administering artesunate in combination with paclitaxel and carboplatin was determined. The activity of artesunate was also assessed in 3D organoid models of primary ovarian cancer and RNAseq analysis was utilized to identify genes and the associated genetic pathways that were differentially regulated in artesunate resistant organoid models compared to organoids that were sensitive to artesunate.
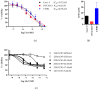
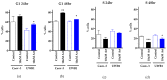
Results: Artesunate treatment reduces cell viability in 2D and 3D ovarian cancer cell models. Clinically relevant concentrations of artesunate induce G1 arrest, but do not induce DNA damage. Pathways related to cell cycle progression, specifically G1/S transition, are upregulated in ovarian organoid models that are innately more resistant to artesunate compared to more sensitive models. Depending on the sequence of administration, the addition of artesunate to carboplatin and paclitaxel improves their effectiveness.
Conclusions: Artesunate has preclinical activity in ovarian cancer that merits further investigation to treat ovarian cancer.
Keywords: Artemesia annua; artesunate; carboplatin; dihydroartemisinin; ovarian cancer; paclitaxel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- American Cancer Society Cancer Statistics Center. [(accessed on 23 November 2020)];2021 Available online: http://cancerstatisticscenter.cancer.org.
-
- McGuire W.P., Hoskins W.J., Brady M.F., Kucera P.R., Partridge E.E., Look K.Y., Clarke-Pearson D.L., Davidson M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. - DOI - PubMed
-
- Ozols R.F., Bundy B.N., Greer B.E., Fowler J.M., Clarke-Pearson D., Burger R.A., Mannel R.S., DeGeest K., Hartenbach E.M., Baergen R. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
-
- Bookman M.A. GOG0182-ICON5: 5-arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. J. Clin. Oncol. 2006;24:5002. doi: 10.1200/jco.2006.24.18_suppl.5002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

