Patient safety and public health concerns: poor dissolution rate of pioglitazone tablets obtained from China, Myanmar and internet sites
- PMID: 33653417
- PMCID: PMC7923830
- DOI: 10.1186/s40360-021-00478-x
Patient safety and public health concerns: poor dissolution rate of pioglitazone tablets obtained from China, Myanmar and internet sites
Abstract
Background: Poor quality medicines have serious implications for public health. The aim of this study was to explore the quality of the antidiabetic pioglitazone, using samples collected in China and Myanmar, and samples purchased online.
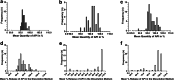
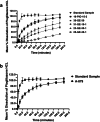
Methods: In this cross-sectional study, we examined samples (n = 163) collected from hospitals in Shanghai, China in 2012 (n = 44), products purchased via the internet and imported into Japan in 2013 (n = 59), and samples purchased in shops in Yangon, Myanmar in 2015 (n = 60). Collected samples were subjected to visual inspection, authenticity investigation and quality testing (potency, content uniformity and dissolution test) by high-performance liquid chromatography. Samples were rated as compliant or non-compliant based on the relevant pharmacopoeial acceptance criteria.
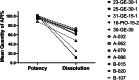
Results: Visual inspection of all samples revealed compliant products. However, responses from manufacturers during authenticity investigation were poor. Among the n = 44 samples from China, one was non-compliant in the potency test. Among the n = 59 samples personally imported into Japan, 38% of generic samples were found to be non-compliant. In Myanmar, 13.3% of samples were non-compliant. Non-compliant samples predominantly failed in the dissolution test. All non-compliant samples were generic.
Conclusions: Despite the apparent satisfactory outcome on the samples from China, pioglitazone samples collected in Myanmar and purchased online for personal import into Japan included many substandard products, which failed quality assessment predominantly because of poor dissolution. Internet providers did not comply with Japanese regulations in various respects.
Keywords: Antidiabetic; Medicine; Pharmacoepidemiology; Pioglitazone; Quality; Substandard.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Quality of omeprazole purchased via the Internet and personally imported into Japan: comparison with products sampled in other Asian countries.Trop Med Int Health. 2018 Mar;23(3):263-269. doi: 10.1111/tmi.13028. Epub 2018 Jan 21. Trop Med Int Health. 2018. PMID: 29314458
-
Quality and Authenticity of Metformin Tablets Circulating on Japanese Websites.Ther Innov Regul Sci. 2021 Jul;55(4):656-666. doi: 10.1007/s43441-021-00262-3. Epub 2021 Feb 17. Ther Innov Regul Sci. 2021. PMID: 33595786 Free PMC article.
-
Circulation of COVID-19-Related Medicines on Japanese Websites during the COVID-19 Pandemic and Their Quality and Authenticity.Am J Trop Med Hyg. 2024 Sep 17;111(5):1097-1106. doi: 10.4269/ajtmh.23-0710. Print 2024 Nov 6. Am J Trop Med Hyg. 2024. PMID: 39288767 Free PMC article.
-
Quality analytics of Internet pharmaceuticals.Anal Bioanal Chem. 2010 Sep;398(1):125-36. doi: 10.1007/s00216-010-3912-4. Epub 2010 Jun 27. Anal Bioanal Chem. 2010. PMID: 20582403 Review.
-
[Trends in the quality evaluation of generic products and bioequivalence guidelines].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2012;(130):1-12. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2012. PMID: 23243982 Review. Japanese.
Cited by
-
Quality of dorzolamide hydrochloride and timolol maleate containing eye drops distributed online.Saudi Pharm J. 2023 Jun;31(6):921-928. doi: 10.1016/j.jsps.2023.04.018. Epub 2023 Apr 24. Saudi Pharm J. 2023. PMID: 37250359 Free PMC article.
References
-
- WHO . Definitions of substandard and falsified (SF) medical products. 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

