SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2): diagnostic accuracy study
- PMID: 33653694
- PMCID: PMC7921617
- DOI: 10.1136/bmj.n423
SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2): diagnostic accuracy study
Abstract
Objective: To evaluate the performance of new lateral flow immunoassays (LFIAs) suitable for use in a national coronavirus disease 2019 (covid-19) seroprevalence programme (real time assessment of community transmission 2-React 2).
Design: Diagnostic accuracy study.
Setting: Laboratory analyses were performed in the United Kingdom at Imperial College, London and university facilities in London. Research clinics for finger prick sampling were run in two affiliated NHS trusts.
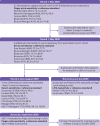
Participants: Sensitivity analyses were performed on sera stored from 320 previous participants in the React 2 programme with confirmed previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Specificity analyses were performed on 1000 prepandemic serum samples. 100 new participants with confirmed previous SARS-CoV-2 infection attended study clinics for finger prick testing.
Interventions: Laboratory sensitivity and specificity analyses were performed for seven LFIAs on a minimum of 200 serum samples from participants with confirmed SARS-CoV-2 infection and 500 prepandemic serum samples, respectively. Three LFIAs were found to have a laboratory sensitivity superior to the finger prick sensitivity of the LFIA currently used in React 2 seroprevalence studies (84%). These LFIAs were then further evaluated through finger prick testing on participants with confirmed previous SARS-CoV-2 infection: two LFIAs (Surescreen, Panbio) were evaluated in clinics in June-July 2020 and the third LFIA (AbC-19) in September 2020. A spike protein enzyme linked immunoassay and hybrid double antigen binding assay were used as laboratory reference standards.
Main outcome measures: The accuracy of LFIAs in detecting immunoglobulin G (IgG) antibodies to SARS-CoV-2 compared with two reference standards.
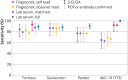
Results: The sensitivity and specificity of seven new LFIAs that were analysed using sera varied from 69% to 100%, and from 98.6% to 100%, respectively (compared with the two reference standards). Sensitivity on finger prick testing was 77% (95% confidence interval 61.4% to 88.2%) for Panbio, 86% (72.7% to 94.8%) for Surescreen, and 69% (53.8% to 81.3%) for AbC-19 compared with the reference standards. Sensitivity for sera from matched clinical samples performed on AbC-19 was significantly higher with serum than finger prick at 92% (80.0% to 97.7%, P=0.01). Antibody titres varied considerably among cohorts. The numbers of positive samples identified by finger prick in the lowest antibody titre quarter varied among LFIAs.
Conclusions: One new LFIA was identified with clinical performance suitable for potential inclusion in seroprevalence studies. However, none of the LFIAs tested had clearly superior performance to the LFIA currently used in React 2 seroprevalence surveys, and none showed sufficient sensitivity and specificity to be considered for routine clinical use.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Department of Health and Social Care, NIHR Biomedical Research Centre of Imperial College NHS Trust, UK Research and Innovation/Medical Research Council, Francis Crick Institute, Cancer Research UK, the Wellcome Trust, Huo Family Foundation and Imperial College London for the submitted work; PC, MM, and RT hold intellectual property rights on the hybrid DABA; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. ENE-COVID Study Group . Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020;396:535-44. https://linkinghub.elsevier.com/retrieve/pii/S0140673620314835. 10.1016/S0140-6736(20)31483-5 - DOI - PMC - PubMed
-
- Ward H, Atchinson JC, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100 000 adults. MedRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.08.12.20173690v2 10.1101/2020.08.12.20173690 - DOI
-
- Ward H, Cooke G, Atchinson C, et al. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365 000 adults. medRxiv. 2020.
-
- Flower B, Brown JC, Simmons B, et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. 2020. https://thorax.bmj.com/lookup/doi/10.1136/thoraxjnl-2020-215732 - DOI - PMC - PubMed
-
- Atchinson C, Pristerà P, Cooper E, et al. Usability and acceptability of home-based self-testing for SARS-CoV-2 antibodies for population surveillance. 2020. http://fdslive.oup.com/www.oup.com/pdf/production_in_progress.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
