Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study
- PMID: 33653802
- PMCID: PMC7929866
- DOI: 10.1136/jitc-2020-001941
Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study
Abstract
Background: Although intravesical BCG is the standard treatment of high-grade non-muscle invasive bladder cancer (NMIBC), response rates remain unsatisfactory. In preclinical models, rapamycin enhances BCG vaccine efficacy against tuberculosis and the killing capacity of γδ T cells, which are critical for BCG's antitumor effects. Here, we monitored immunity, safety, and tolerability of rapamycin combined with BCG in patients with NMIBC.
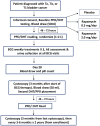
Methods: A randomized double-blind trial of oral rapamycin (0.5 or 2.0 mg daily) versus placebo for 1 month was conducted in patients with NMIBC concurrently receiving 3 weekly BCG instillations (NCT02753309). The primary outcome was induction of BCG-specific γδ T cells, measured as a percentage change from baseline. Post-BCG urinary cytokines and immune cells were examined as surrogates for local immune response in the bladder. Secondary outcomes measured were adverse events (AEs) and tolerability using validated patient-reported questionnaires.
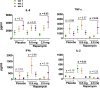
Results: Thirty-one patients were randomized (11 placebo, 8 rapamycin 2.0 mg, and 12 rapamycin 0.5 mg). AEs were similar across groups and most were grade 1-2. One (12.5%) patient randomized to 2.0 mg rapamycin was taken off treatment due to stomatitis. No significant differences in urinary symptoms, bowel function, or bother were observed between groups. The median (IQR) percentage change in BCG-specific γδ T cells from baseline per group was as follows: -26% (-51% to 24%) for placebo, 9.6% (-59% to 117%) for rapamycin 0.5 mg (versus placebo, p=0.18), and 78.8% (-31% to 115%) for rapamycin 2.0 mg (versus placebo, p=0.03). BCG-induced cytokines showed a progressive increase in IL-8 (p=0.02) and TNF-α (p=0.04) over time for patients on rapamycin 2.0 mg, whereas patients receiving placebo had no significant change in urinary cytokines. Compared with placebo, patients receiving 2.0 mg rapamycin had increased urinary γδ T cells at the first week of BCG (p=0.02).
Conclusions: Four weeks of 0.5 and 2.0 mg oral rapamycin daily is safe and tolerable in combination with BCG for patients with NMIBC. Rapamycin enhances BCG-specific γδ T cell immunity and boosts urinary cytokines during BCG treatment. Further study is needed to determine long-term rapamycin safety, tolerability and effects on BCG efficacy.
Keywords: immunity; immunotherapy; innate; urinary bladder neoplasms.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The studies related to this manuscript were conducted abided by research ethics and approved by IRB guidelines for clinical sample use. All authors have consent for publishing the data from the studies. RS discloses other roles as Consultant for FerGene and Clinical Research Support for JBL(SWOG), FKD and Decipher Biosciences. All other authors disclose no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
