Electrocardiographic features of immune checkpoint inhibitor associated myocarditis
- PMID: 33653803
- PMCID: PMC7929895
- DOI: 10.1136/jitc-2020-002007
Electrocardiographic features of immune checkpoint inhibitor associated myocarditis
Abstract
Background: Myocarditis is a highly morbid complication of immune checkpoint inhibitor (ICI) use that remains inadequately characterized. The QRS duration and the QTc interval are standardized electrocardiographic measures that are prolonged in other cardiac conditions; however, there are no data on their utility in ICI myocarditis.
Methods: From an international registry, ECG parameters were compared between 140 myocarditis cases and 179 controls across multiple time points (pre-ICI, on ICI prior to myocarditis, and at the time of myocarditis). The association between ECG values and major adverse cardiac events (MACE) was also tested.
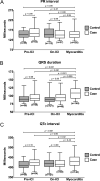
Results: Both the QRS duration and QTc interval were similar between cases and controls prior to myocarditis. When compared with controls on an ICI (93±19 ms) or to baseline prior to myocarditis (97±19 ms), the QRS duration prolonged with myocarditis (110±22 ms, p<0.001 and p=0.009, respectively). In contrast, the QTc interval at the time of myocarditis (435±39 ms) was not increased compared with pre-myocarditis baseline (422±27 ms, p=0.42). A prolonged QRS duration conferred an increased risk of subsequent MACE (HR 3.28, 95% CI 1.98 to 5.62, p<0.001). After adjustment, each 10 ms increase in the QRS duration conferred a 1.3-fold increase in the odds of MACE (95% CI 1.07 to 1.61, p=0.011). Conversely, there was no association between the QTc interval and MACE among men (HR 1.33, 95% CI 0.70 to 2.53, p=0.38) or women (HR 1.48, 95% CI 0.61 to 3.58, p=0.39).
Conclusions: The QRS duration is increased in ICI myocarditis and is associated with increased MACE risk. Use of this widely available ECG parameter may aid in ICI myocarditis diagnosis and risk-stratification.
Keywords: autoimmunity; immune tolerance; immunotherapy; inflammation; self tolerance.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SSM has received consultancy fees from OMR Globus, Alpha Detail, and Opinion Research Team. RS has been a consultant to Merck and Novartis. LH has received consultancy, advisory board, and speaker fees from MSD, BMS, Roche, Novartis, Amgen, and Curevac. LZu has been a consultant to Merck. JG has received research support from Amgen. AN has received research support from Amgen and has been a consultant for Takeda Oncology and AstraZeneca. TGN has received advisory fees from Parexel, AbbVie, H3-Biomedicine, Aprea Therapeutics, BMS, and Intrinsic Imaging. TGN has received grant funding from AstraZeneca.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
