The prevalence of antibodies to SARS-CoV-2 among blood donors in China
- PMID: 33654063
- PMCID: PMC7925561
- DOI: 10.1038/s41467-021-21503-x
The prevalence of antibodies to SARS-CoV-2 among blood donors in China
Abstract
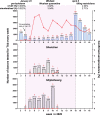
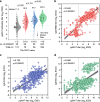
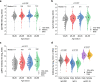
In this study, we investigate the seroprevalence of SARS-CoV-2 antibodies among blood donors in the cities of Wuhan, Shenzhen, and Shijiazhuang in China. From January to April 2020, 38,144 healthy blood donors in the three cities were tested for total antibody against SARS-CoV-2 followed by pseudotype SARS-CoV-2 neutralization tests, IgG, and IgM antibody testing. Finally, a total of 398 donors were confirmed positive. The age- and sex-standardized SARS-CoV-2 seroprevalence among 18-60 year-old adults (18-65 year-old in Shenzhen) was 2.66% (95% CI: 2.24%-3.07%) in Wuhan, 0.033% (95% CI: 0.0029%-0.267%) in Shenzhen, and 0.0028% (95% CI: 0.0001%-0.158%) in Shijiazhuang, respectively. Female sex and older-age were identified to be independent risk factors for SARS-CoV-2 seropositivity among blood donors in Wuhan. As most of the population of China remained uninfected during the early wave of the COVID-19 pandemic, effective public health measures are still certainly required to block viral spread before a vaccine is widely available.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures

References
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-genera... (2020).
-
- World Health Organization. Coronavirus disease (COVID-19) outbreak situation 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

