Mice lacking PLAP-1/asporin counteracts high fat diet-induced metabolic disorder and alveolar bone loss by controlling adipose tissue expansion
- PMID: 33654143
- PMCID: PMC7925592
- DOI: 10.1038/s41598-021-84512-2
Mice lacking PLAP-1/asporin counteracts high fat diet-induced metabolic disorder and alveolar bone loss by controlling adipose tissue expansion
Abstract
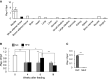
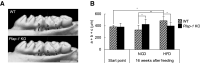
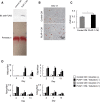
Adipose tissue fibrosis with chronic inflammation is a hallmark of obesity-related metabolic disorders, and the role of proteoglycans in developing adipose tissue fibrosis is of interest. Periodontal disease is associated with obesity; however, the underlying molecular mechanisms remain unclear. Here we investigated the roles of periodontal ligament associated protein-1 (PLAP-1)/asporin, a proteoglycan preferentially and highly expressed in the periodontal ligament, in obesity-related adipose tissue dysfunction and adipocyte differentiation. It was found that PLAP-1 is also highly expressed in white adipose tissues. Plap-1 knock-out mice counteracted obesity and alveolar bone resorption induced by a high-fat diet. Plap-1 knock-down in 3T3-L1 cells resulted in less lipid accumulation, and recombinant PLAP-1 enhanced lipid accumulation in 3T3-L1 cells. In addition, it was found that primary preadipocytes isolated from Plap-1 knock-out mice showed lesser lipid accumulation than the wild-type (WT) mice. Furthermore, the stromal vascular fraction of Plap-1 knock-out mice showed different extracellular matrix gene expression patterns compared to WT. These findings demonstrate that PLAP-1 enhances adipogenesis and could be a key molecule in understanding the association between periodontal disease and obesity-related metabolic disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis.Int J Mol Sci. 2023 Nov 5;24(21):15989. doi: 10.3390/ijms242115989. Int J Mol Sci. 2023. PMID: 37958972 Free PMC article.
-
Periodontal ligament-associated protein-1 knockout mice regulate the differentiation of osteoclasts and osteoblasts through TGF-β1/Smad signaling pathway.J Cell Physiol. 2024 Mar;239(3):e31062. doi: 10.1002/jcp.31062. Epub 2023 Jun 25. J Cell Physiol. 2024. PMID: 37357387
-
PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization.J Biol Chem. 2007 Aug 10;282(32):23070-80. doi: 10.1074/jbc.M611181200. Epub 2007 May 23. J Biol Chem. 2007. PMID: 17522060
-
PLAP-1/Asporin Regulates TLR2- and TLR4-induced Inflammatory Responses.J Dent Res. 2015 Dec;94(12):1706-14. doi: 10.1177/0022034515606859. Epub 2015 Sep 23. J Dent Res. 2015. PMID: 26399972
-
The multifaceted progenitor fates in healthy or unhealthy adipose tissue during obesity.Rev Endocr Metab Disord. 2021 Dec;22(4):1111-1119. doi: 10.1007/s11154-021-09662-0. Epub 2021 Jun 8. Rev Endocr Metab Disord. 2021. PMID: 34105090 Review.
Cited by
-
Whole Exome Sequencing to Find Candidate Variants for the Prediction of Kidney Transplantation Efficacy.Genes (Basel). 2023 Jun 11;14(6):1251. doi: 10.3390/genes14061251. Genes (Basel). 2023. PMID: 37372431 Free PMC article.
-
Small Extracellular Vesicles in Milk Cross the Blood-Brain Barrier in Murine Cerebral Cortex Endothelial Cells and Promote Dendritic Complexity in the Hippocampus and Brain Function in C57BL/6J Mice.Front Nutr. 2022 May 6;9:838543. doi: 10.3389/fnut.2022.838543. eCollection 2022. Front Nutr. 2022. PMID: 35600828 Free PMC article.
-
Unraveling adipose tissue proteomic landscapes in severe obesity: insights into metabolic complications and potential biomarkers.Am J Physiol Endocrinol Metab. 2023 Nov 1;325(5):E562-E580. doi: 10.1152/ajpendo.00153.2023. Epub 2023 Oct 4. Am J Physiol Endocrinol Metab. 2023. PMID: 37792298 Free PMC article.
-
The Potential of miR-21 in Stem Cell Differentiation and its Application in Tissue Engineering and Regenerative Medicine.Stem Cell Rev Rep. 2023 Jul;19(5):1232-1251. doi: 10.1007/s12015-023-10510-8. Epub 2023 Mar 11. Stem Cell Rev Rep. 2023. PMID: 36899116 Review.
-
ASPN Synergizes with HAPLN1 to Inhibit the Osteogenic Differentiation of Bone Marrow Mesenchymal Stromal Cells and Extracellular Matrix Mineralization of Osteoblasts.Orthop Surg. 2023 Sep;15(9):2423-2434. doi: 10.1111/os.13803. Epub 2023 Jul 10. Orthop Surg. 2023. PMID: 37427673 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

