MAPRE2 mutations result in altered human cranial neural crest migration, underlying craniofacial malformations in CSC-KT syndrome
- PMID: 33654163
- PMCID: PMC7925611
- DOI: 10.1038/s41598-021-83771-3
MAPRE2 mutations result in altered human cranial neural crest migration, underlying craniofacial malformations in CSC-KT syndrome
Abstract
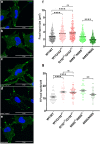
Circumferential skin creases (CSC-KT) is a rare polymalformative syndrome characterised by intellectual disability associated with skin creases on the limbs, and very characteristic craniofacial malformations. Previously, heterozygous and homozygous mutations in MAPRE2 were found to be causal for this disease. MAPRE2 encodes for a member of evolutionary conserved microtubule plus end tracking proteins, the end binding (EB) family. Unlike MAPRE1 and MAPRE3, MAPRE2 is not required for the persistent growth and stabilization of microtubules, but plays a role in other cellular processes such as mitotic progression and regulation of cell adhesion. The mutations identified in MAPRE2 all reside within the calponin homology domain, responsible to track and interact with the plus-end tip of growing microtubules, and previous data showed that altered dosage of MAPRE2 resulted in abnormal branchial arch patterning in zebrafish. In this study, we developed patient derived induced pluripotent stem cell lines for MAPRE2, together with isogenic controls, using CRISPR/Cas9 technology, and differentiated them towards neural crest cells with cranial identity. We show that changes in MAPRE2 lead to alterations in neural crest migration in vitro but also in vivo, following xenotransplantation of neural crest progenitors into developing chicken embryos. In addition, we provide evidence that changes in focal adhesion might underlie the altered cell motility of the MAPRE2 mutant cranial neural crest cells. Our data provide evidence that MAPRE2 is involved in cellular migration of cranial neural crest and offers critical insights into the mechanism underlying the craniofacial dysmorphisms and cleft palate present in CSC-KT patients. This adds the CSC-KT disorder to the growing list of neurocristopathies.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

