A single-chain antibody generation system yielding CAR-T cells with superior antitumor function
- PMID: 33654176
- PMCID: PMC7925539
- DOI: 10.1038/s42003-021-01791-1
A single-chain antibody generation system yielding CAR-T cells with superior antitumor function
Abstract
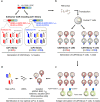
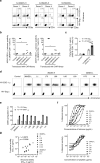
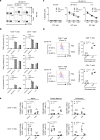
Cancer immunotherapy using T cells redirected with chimeric antigen receptor (CAR) has shown a lot of promise. We have established a single-chain antibody (scFv) generation system in which scFv library-expressing CAR-T cells can be screened appropriately based on their antitumor functions. A variable region library containing the variable and J regions of the human immunoglobulin light or heavy chain was fused with the variable region of a heavy or light chain encoded by an existing tumor-specific antibody to generate a new scFv library. Then, scFv library-expressing CAR-T cells were generated and stimulated with target cells to concentrate the antigen-specific population. Using this system, target-specific recognition of CAR-T cells appeared to be finely tuned by selecting a new variable region. Importantly, we have demonstrated that the newly optimized scFv-expressing CAR-T cells had better proliferation capacity and durable phenotypes, enabling superior reactivity against advanced tumors in vivo in comparison with the original CAR-T cells. Therefore, the optimization of an scFv is needed to maximize the in vivo antitumor functions of CAR-T cells. This system may allow us to adjust an immunological synapse formed by an scFv expressed by CAR-T cells and a target antigen, representing an ideal form of CAR-T-cell immunotherapy.
Conflict of interest statement
Ehime University has filed a patent application related to this study (WO 2020/162452) on which T.O., H.F., K. Takenaka, and M.Y. are named as inventors. T.O. has ownership interest in Optieum Biotechnologies Inc.. None of the other authors have any conflicts of interest to disclose.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

