Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: the Alliance CALGB 10603/RATIFY trial
- PMID: 33654204
- PMCID: PMC8591906
- DOI: 10.1038/s41375-021-01179-4
Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: the Alliance CALGB 10603/RATIFY trial
Abstract
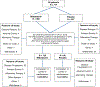
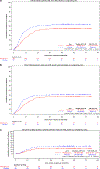
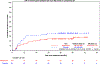
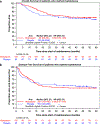
The prospective randomized, placebo-controlled CALGB 10603/RATIFY trial (Alliance) demonstrated a statistically significant overall survival benefit from the addition of midostaurin to standard frontline chemotherapy in a genotypically-defined subgroup of 717 patients with FLT3-mutant acute myeloid leukemia (AML). The risk of death was reduced by 22% on the midostaurin-containing arm. In this post hoc analysis, we analyzed the cumulative incidence of relapse (CIR) on this study and also evaluated the impact of 12 4-week cycles of maintenance therapy. CIR analyses treated relapses and AML deaths as events, deaths from other causes as competing risks, and survivors in remission were censored. CIR was improved on the midostaurin arm (HR = 0.71 (95% CI, 0.54-0.93); p = 0.01), both overall and within European LeukemiaNet 2017 risk classification subsets when post-transplant events were considered in the analysis as events. However, when transplantation was considered as a competing risk, there was overall no significant difference between the risks of relapse on the two randomized arms. Patients still in remission after consolidation with high-dose cytarabine entered the maintenance phase, continuing with either midostaurin or placebo. Analyses were inconclusive in quantifying the impact of the maintenance phase on the overall outcome. In summary, midostaurin reduces the CIR.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited part of Springer Nature.
Figures


References
-
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10(12): 1911–1918. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759. - PubMed
-
- Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335. - PubMed
-
- Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res 2001; 61: 7233–7239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA032291/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- P50 CA206963/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233338/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

