Electroimmunology and cardiac arrhythmia
- PMID: 33654273
- PMCID: PMC9703448
- DOI: 10.1038/s41569-021-00520-9
Electroimmunology and cardiac arrhythmia
Abstract
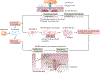
Conduction disorders and arrhythmias remain difficult to treat and are increasingly prevalent owing to the increasing age and body mass of the general population, because both are risk factors for arrhythmia. Many of the underlying conditions that give rise to arrhythmia - including atrial fibrillation and ventricular arrhythmia, which frequently occur in patients with acute myocardial ischaemia or heart failure - can have an inflammatory component. In the past, inflammation was viewed mostly as an epiphenomenon associated with arrhythmia; however, the recently discovered inflammatory and non-canonical functions of cardiac immune cells indicate that leukocytes can be arrhythmogenic either by altering tissue composition or by interacting with cardiomyocytes; for example, by changing their phenotype or perhaps even by directly interfering with conduction. In this Review, we discuss the electrophysiological properties of leukocytes and how these cells relate to conduction in the heart. Given the thematic parallels, we also summarize the interactions between immune cells and neural systems that influence information transfer, extrapolating findings from the field of neuroscience to the heart and defining common themes. We aim to bridge the knowledge gap between electrophysiology and immunology, to promote conceptual connections between these two fields and to explore promising opportunities for future research.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
M.N. has received funds or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis and Pfizer, as well as consulting fees from Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, Takeda and Verseau Therapeutics. The other authors declare no competing interests.
Figures


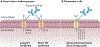
References
-
- Byrne R et al. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the West of Ireland. Eur. Heart J 29, 1418–1423 (2008). - PubMed
-
- Dalia AA et al. A narrative review for anesthesiologists of the 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J. Cardiothorac. Vasc. Anesth 33, 1722–1730 (2019). - PubMed
-
- Vaillancourt C, Stiell IG & Canadian Cardiovascular Outcomes Research Team. Cardiac arrest care and emergency medical services in Canada. Can. J. Cardiol 20, 1081–1090 (2004). - PubMed
-
- Chugh SS et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large US community. J. Am. Coll. Cardiol 44, 1268–1275 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

