Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide
- PMID: 33654306
- PMCID: PMC8903027
- DOI: 10.1038/s41571-021-00479-z
Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide
Abstract
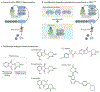
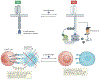
For decades, anticancer targeted therapies have been designed to inhibit kinases or other enzyme classes and have profoundly benefited many patients. However, novel approaches are required to target transcription factors, scaffolding proteins and other proteins central to cancer biology that typically lack catalytic activity and have remained mostly recalcitrant to drug development. The selective degradation of target proteins is an attractive approach to expand the druggable proteome, and the selective oestrogen receptor degrader fulvestrant served as an early example of this concept. Following a long and tragic history in the clinic, the immunomodulatory imide drug (IMiD) thalidomide was discovered to exert its therapeutic activity via a novel and unexpected mechanism of action: targeting proteins to an E3 ubiquitin ligase for subsequent proteasomal degradation. This discovery has paralleled and directly catalysed myriad breakthroughs in drug development, leading to the rapid maturation of generalizable chemical platforms for the targeted degradation of previously undruggable proteins. Decades of clinical experience have established front-line roles for thalidomide analogues, including lenalidomide and pomalidomide, in the treatment of haematological malignancies. With a new generation of 'degrader' drugs currently in development, this experience provides crucial insights into class-wide features of degraders, including a unique pharmacology, mechanisms of resistance and emerging therapeutic opportunities. Herein, we review these past experiences and discuss their application in the clinical development of novel degrader therapies.
Figures


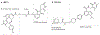
References
-
- Stephens T & Brynner R Dark Remedy: The Impact of Thalidomide and its Revival as a Vital Medicine. (Perseus Publishing, 2009).
-
- Lenz W, Pfeiffer RA, Kosenow W & Hayman DJ THALIDOMIDE AND CONGENITAL ABNORMALITIES. Lancet 279, 45–46 (1962).
-
- Mcbride WG THALIDOMIDE AND CONGENITAL ABNORMALITIES. Lancet 278, 1358 (1961).
-
- Mintz M “Heroine” of FDA Keeps Bad Drug Off of Market. The Washington Post (1962).
-
- Kaur I, Dogra S, Narang T & De D Comparative efficacy of thalidomide and prednisolone in the treatment of moderate to severe erythema nodosum leprosum: A randomized study. Australas J Dermatol 50, 181–185 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

