Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET)
- PMID: 33654881
- PMCID: PMC7853939
- DOI: 10.21769/BioProtoc.3385
Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET)
Abstract
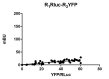
Detecting protein-protein interactions by co-immunoprecipitation provided a major advancement in the immunology research field. In the G-protein-coupled receptors (GPCRs) research field, colocalization and co-immunoprecipitation were used to detect interactions, but doubts arose due to specificity of the antibodies (monoclonal in the case of receptors related to immunology and polyclonal in the case of GPCRs) and due to the possibility of false positive due to the potential occurrence of bridging proteins. Accordingly, new methodological approaches were needed, and energy transfer techniques have been instrumental to detect direct protein-protein, protein-receptor or receptor-receptor interactions. Of the two most relevant methods (Förster, or fluorescence resonance energy transfer: FRET and Bioluminescence energy transfer: BRET), the protocol for BRET is here presented. BRET has been instrumental to detect direct interactions between GPCRs and has contributed to demonstrate that GPCR dimers/oligomer functionality is different from that exerted by individual receptors. Advantages outweigh those of FRET as no fluorescence source is needed. Interestingly, BRET is not only useful to validate interactions detected by other means or hypothesized in the basis of indirect evidence, but to measure signal transduction events. In fact, BRET may, for instance, be used to assess β-arrestin recruitment to activated GPCRs.
Keywords: BRET; Bioluminescence; Energy transfer; Fluorescence; G-protein-coupled receptors; GPCR; Heteromers; Protein-protein interactions.
Copyright © 2019 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsAuthors declare no competing of interests.
Figures



References
-
- Beautrait A., Michalski K. R., Lopez T. S., Mannix K. M., McDonald D. J., Cutter A. R., Medina C. B., Hebert A. M., Francis C. J., Bouvier M., Tesmer J. J. and Sterne-Marr R.(2014). Mapping the putative G protein-coupled receptor(GPCR) docking site on GPCR kinase 2: insights from intact cell phosphorylation and recruitment assays. J Biol Chem 289(36): 25262-25275. - PMC - PubMed
-
- Burger R., Schrod L. and Schaefer H.(1986). Functionally relevant membrane proteins of human and guinea-pig T lymphocytes. Mol Immunol 23(11): 1149-1156. - PubMed
-
- Canals M., Burgueno J., Marcellino D., Cabello N., Canela E. I., Mallol J., Agnati L., Ferre S., Bouvier M., Fuxe K., Ciruela F., Lluis C. and Franco R.(2004). Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer . J Neurochem 88(3): 726-734. - PubMed
-
- Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S. R., Neve K., Fuxe K., Agnati L. F., Woods A. S., Ferre S., Lluis C., Bouvier M. and Franco R.(2003). Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer . J Biol Chem 278(47): 46741-46749. - PubMed
LinkOut - more resources
Full Text Sources

