Herpes Simplex Virus Type 1 Propagation, Titration and Single-step Growth Curves
- PMID: 33654936
- PMCID: PMC7853997
- DOI: 10.21769/BioProtoc.3441
Herpes Simplex Virus Type 1 Propagation, Titration and Single-step Growth Curves
Abstract
Given the endemic seroprevalence of herpes simplex viruses (HSV), its associated human diseases, and the emergence of acyclovir-resistant strains, there is a continuous need for better antiviral therapies. Towards this aim, identifying mechanistic details of how HSV-1 manipulates infected cells, how it modulates the immune responses, and how it causes diseases are essential. Measuring titers and growth kinetics of clinical isolates and viral mutants are important for a thorough characterization of viral phenotypes in vitro and in vivo. We provide protocols for the preparation as well as titration of HSV-1 stocks, and explain how to perform single-step growth curves to characterize the functions of viral proteins or host factors during infection. In particular, we describe methods to prepare and characterize high-titer HSV-1 stocks with low genome to titer ratios that are required for infection studies in cell culture and animal experiments.
Keywords: Herpes simplex virus type 1; Plaque assay; Single-step growth curve; Virus propagation.
Copyright © 2019 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

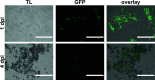



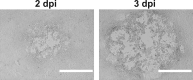
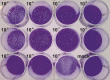

References
-
- Coffin R. S., MacLean A. R., Latchman D. S. and Brown S. M.(1996). Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther 3(10): 886-891. - PubMed
-
- Coffin R. S., Thomas S. K., Thomas N. S., Lilley C. E., Pizzey A. R., Griffiths C. H., Gibb B. J., Wagstaff M. J., Inges S. J., Binks M. H., Chain B. M., Thrasher A. J., Rutault K. and Latchman D. S.(1998). Pure populations of transduced primary human cells can be produced using GFP expressing herpes virus vectors and flow cytometry. Gene Ther 5(5): 718-722. - PubMed
-
- Diefenbach R. J., Miranda-Saksena M., Douglas M. W. and Cunningham A. L.(2008). Transport and egress of herpes simplex virus in neurons. Rev Med Virol 18(1): 35-51. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

