Differentiation of Human Induced Pluripotent Stem Cells (iPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons
- PMID: 33654990
- PMCID: PMC7854068
- DOI: 10.21769/BioProtoc.3188
Differentiation of Human Induced Pluripotent Stem Cells (iPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons
Abstract
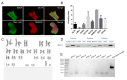
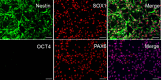
Induced Pluripotent Stem Cells (iPSCs) are pluripotent stem cells that can be generated from somatic cells, and provide a way to model the development of neural tissues in vitro. One particularly interesting application of iPSCs is the development of neurons analogous to those found in the human forebrain. Forebrain neurons play a central role in cognition and sensory processing, and deficits in forebrain neuronal activity contributes to a host of conditions, including epilepsy, Alzheimer's disease, and schizophrenia. Here, we present our protocol for differentiating iPSCs into forebrain neural progenitor cells (NPCs) and neurons, whereby neural rosettes are generated from stem cells without dissociation and NPCs purified from rosettes based on their adhesion, resulting in a more rapid generation of pure NPC cultures. Neural progenitor cells can be maintained as long-term cultures, or differentiated into forebrain neurons. This protocol provides a simplified and fast methodology of generating forebrain NPCs and neurons, and enables researchers to generate effective in vitro models to study forebrain disease and neurodevelopment. This protocol can also be easily adapted to generate other neural lineages.
Keywords: Cortical; Forebrain; NPC; NSC; Neuron; iPSC.
Copyright © 2019 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsCarl Ernst is president of ManuStem.com.
Figures




Similar articles
-
Highly efficient methods to obtain homogeneous dorsal neural progenitor cells from human and mouse embryonic stem cells and induced pluripotent stem cells.Stem Cell Res Ther. 2018 Mar 15;9(1):67. doi: 10.1186/s13287-018-0812-6. Stem Cell Res Ther. 2018. PMID: 29544541 Free PMC article.
-
Rapid and Simplified Induction of Neural Stem/Progenitor Cells (NSCs/NPCs) and Neurons from Human Induced Pluripotent Stem Cells (hiPSCs).Bio Protoc. 2021 Feb 5;11(3):e3914. doi: 10.21769/BioProtoc.3914. eCollection 2021 Feb 5. Bio Protoc. 2021. PMID: 33732801 Free PMC article.
-
Differentiation of Human Induced Pluripotent Stem Cells into Cortical Neurons to Advance Precision Medicine.Methods Mol Biol. 2022;2429:143-174. doi: 10.1007/978-1-0716-1979-7_10. Methods Mol Biol. 2022. PMID: 35507160
-
Applications of induced pluripotent stem cell technologies in spinal cord injury.J Neurochem. 2017 Jun;141(6):848-860. doi: 10.1111/jnc.13986. Epub 2017 Apr 5. J Neurochem. 2017. PMID: 28199003 Review.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
Comparison of osteoclast differentiation protocols from human induced pluripotent stem cells of different tissue origins.Res Sq [Preprint]. 2023 Jul 7:rs.3.rs-3089289. doi: 10.21203/rs.3.rs-3089289/v1. Res Sq. 2023. Update in: Stem Cell Res Ther. 2023 Nov 7;14(1):319. doi: 10.1186/s13287-023-03547-6. PMID: 37461708 Free PMC article. Updated. Preprint.
-
Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons.Genes (Basel). 2024 May 10;15(5):609. doi: 10.3390/genes15050609. Genes (Basel). 2024. PMID: 38790238 Free PMC article.
-
Robust Expression of Functional NMDA Receptors in Human Induced Pluripotent Stem Cell-Derived Neuronal Cultures Using an Accelerated Protocol.Front Mol Neurosci. 2021 Nov 26;14:777049. doi: 10.3389/fnmol.2021.777049. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34899184 Free PMC article.
-
Modeling HIV-1 infection and NeuroHIV in hiPSCs-derived cerebral organoid cultures.J Neurovirol. 2024 Aug;30(4):362-379. doi: 10.1007/s13365-024-01204-z. Epub 2024 Apr 10. J Neurovirol. 2024. PMID: 38600307 Free PMC article.
-
Plasma-Activated Coated Glass: A Novel Platform for Optimal Optical Performance and Cell Culture Substrate Customization.Small Sci. 2024 Jan 24;4(4):2300228. doi: 10.1002/smsc.202300228. eCollection 2024 Apr. Small Sci. 2024. PMID: 40213000 Free PMC article.
References
-
- Auld D. S., Kornecook T. J., Bastianetto S. and Quirion R.(2002). Alzheimer's disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68(3): 209-245. - PubMed
-
- Baxter M. G. and Chiba A. A.(1999). Cognitive functions of the basal forebrain. Curr Opin Neurobiol 9(2): 178-183. - PubMed
-
- Bell S., Maussion G., Jefri M., Peng H., Theroux J. F., Silveira H., Soubannier V., Wu H., Hu P., Galat E., Torres-Platas S. G., Boudreau-Pinsonneault C., O'Leary L. A., Galat V., Turecki G., Durcan T. M., Fon E. A., Mechawar N. and Ernst C.(2018). Disruption of GRIN2B impairs differentiation in human neurons. Stem Cell Reports 11(1): 183-196. - PMC - PubMed
-
- Chandrasekaran A., Avci H. X., Ochalek A., Rosingh L. N., Molnar K., Laszlo L., Bellak T., Teglasi A., Pesti K., Mike A., Phanthong P., Biro O., Hall V., Kitiyanant N., Krause K. H., Kobolak J. and Dinnyes A.(2017). Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res 25: 139-151. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

