C-H Activation: Toward Sustainability and Applications
- PMID: 33655064
- PMCID: PMC7908034
- DOI: 10.1021/acscentsci.0c01413
C-H Activation: Toward Sustainability and Applications
Abstract
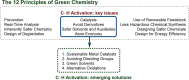
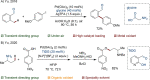
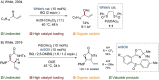
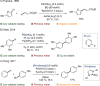
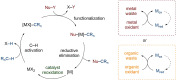
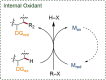
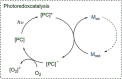
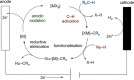
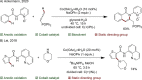
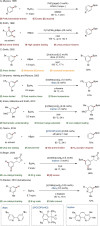
Since the definition of the "12 Principles of Green Chemistry" more than 20 years ago, chemists have become increasingly mindful of the need to conserve natural resources and protect the environment through the judicious choice of synthetic routes and materials. The direct activation and functionalization of C-H bonds, bypassing intermediate functional group installation is, in abstracto, step and atom economic, but numerous factors still hinder the sustainability of large-scale applications. In this Outlook, we highlight the research areas seeking to overcome the sustainability challenges of C-H activation: the pursuit of abundant metal catalysts, the avoidance of static directing groups, the replacement of metal oxidants, and the introduction of bioderived solvents. We close by examining the progress made in the subfield of aryl C-H borylation from its origins, through highly efficient but precious Ir-based systems, to emerging 3d metal catalysts. The future growth of this field will depend on industrial uptake, and thus we urge researchers to strive toward sustainable C-H activation.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



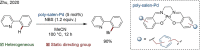

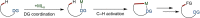


















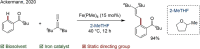





References
-
- Hayler J. D.; Leahy D. K.; Simmons E. M. A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46. 10.1021/acs.organomet.8b00566. - DOI
-
- Laird T. Green Chemistry Is Good Process Chemistry. Org. Process Res. Dev. 2012, 16, 1–2. 10.1021/op200366y. - DOI
-
- Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions: Chemical Strategy for Sustainability Towards a Toxic-Free Environment; Brussels, 2020.
-
- Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/4. Off. J. Eur. Union, 2006.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

