Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study
- PMID: 33655218
- PMCID: PMC7906668
- DOI: 10.1016/S2665-9913(21)00012-6
Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study
Abstract
Background: Patients with severe COVID-19 develop a life-threatening hyperinflammatory response to the virus. Interleukin (IL)-1 or IL-6 inhibitors have been used to treat this patient population, but the comparative effectiveness of these different strategies remains undetermined. We aimed to compare IL-1 and IL-6 inhibition in patients admitted to hospital with COVID-19, respiratory insufficiency, and hyperinflammation.
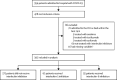
Methods: This cohort study included patients admitted to San Raffaele Hospital (Milan, Italy) with COVID-19, respiratory insufficiency, defined as a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of 300 mm Hg or less, and hyperinflammation, defined as serum C-reactive protein concentration of 100 mg/L or more or ferritin concentration of 900 ng/mL or more. The primary endpoint was survival, and the secondary endpoint was a composite of death or mechanical ventilation (adverse clinical outcome). Multivariable Cox regression analysis was used to compare clinical outcomes of patients receiving IL-1 inhibition (anakinra) or IL-6 inhibition (tocilizumab or sarilumab) with those of patients who did not receive interleukin inhibitors, after accounting for baseline differences. All patients received standard care. Interaction tests were used to assess the probability of survival according to C-reactive protein or lactate dehydrogenase concentrations.
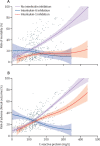
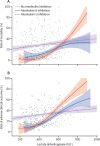
Findings: Of 392 patients included between Feb 25 and May 20, 2020, 275 did not receive interleukin inhibitors, 62 received the IL-1 inhibitor anakinra, and 55 received an IL-6 inhibitor (29 received tocilizumab and 26 received sarilumab). In the multivariable analysis, compared with patients who did not receive interleukin inhibitors, patients treated with IL-1 inhibition had a significantly reduced mortality risk (hazard ratio [HR] 0·450, 95% CI 0·204-0·990, p=0·047), but those treated with IL-6 inhibition did not (0·900, 0·412-1·966; p=0·79). In the multivariable analysis, there was no difference in adverse clinical outcome risk in patients treated with IL-1 inhibition (HR 0·866, 95% CI 0·482-1·553; p=0·63) or IL-6 inhibition (0·882, 0·452-1·722; p=0·71) relative to patients who did not receive interleukin inhibitors. For increasing C-reactive protein concentrations, patients treated with IL-6 inhibition had a significantly reduced risk of mortality (HR 0·990, 95% CI 0·981-0·999; p=0·031) and adverse clinical outcome (0·987, 0·979-0·995; p=0·0021) compared with patients who did not receive interleukin inhibitors. For decreasing concentrations of serum lactate dehydrogenase, patients treated with an IL-1 inhibitor and patients treated with IL-6 inhibitors had a reduced risk of mortality; increasing concentrations of lactate dehydrogenase in patients receiving either interleukin inhibitor were associated with an increased risk of mortality (HR 1·009, 95% CI 1·003-1·014, p=0·0011 for IL-1 inhibitors and 1·006, 1·001-1·011, p=0·028 for IL-6 inhibitors) and adverse clinical outcome (1·006, 1·002-1·010, p=0·0031 for IL-1 inhibitors and 1·005, 1·001-1·010, p=0·016 for IL-6 inhibitors) compared with patients who did not receive interleukin inhibitors.
Interpretation: IL-1 inhibition, but not IL-6 inhibition, was associated with a significant reduction of mortality in patients admitted to hospital with COVID-19, respiratory insufficiency, and hyperinflammation. IL-6 inhibition was effective in a subgroup of patients with markedly high C-reactive protein concentrations, whereas both IL-1 and IL-6 inhibition were effective in patients with low lactate dehydrogenase concentrations.
Funding: None.
© 2021 Elsevier Ltd. All rights reserved.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

