Activation of SnRK2 by Raf-like kinase ARK represents a primary mechanism of ABA and abiotic stress responses
- PMID: 33655297
- PMCID: PMC8133623
- DOI: 10.1093/plphys/kiaa046
Activation of SnRK2 by Raf-like kinase ARK represents a primary mechanism of ABA and abiotic stress responses
Abstract
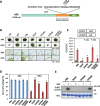
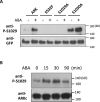
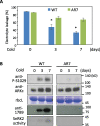
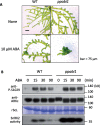
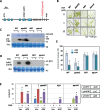
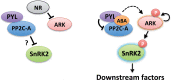
The Raf-like protein kinase abscisic acid (ABA) and abiotic stress-responsive Raf-like kinase (ARK) previously identified in the moss Physcomitrium (Physcomitrella) patens acts as an upstream regulator of subgroup III SNF1-related protein kinase2 (SnRK2), the key regulator of ABA and abiotic stress responses. However, the mechanisms underlying activation of ARK by ABA and abiotic stress for the regulation of SnRK2, including the role of ABA receptor-associated group A PP2C (PP2C-A), are not understood. We identified Ser1029 as the phosphorylation site in the activation loop of ARK, which provided a possible mechanism for regulation of its activity. Analysis of transgenic P. patens ark lines expressing ARK-GFP with Ser1029-to-Ala mutation indicated that this replacement causes reductions in ABA-induced gene expression, stress tolerance, and SnRK2 activity. Immunoblot analysis using an anti-phosphopeptide antibody indicated that ABA treatments rapidly stimulate Ser1029 phosphorylation in the wild type (WT). The phosphorylation profile of Ser1029 in ABA-hypersensitive ppabi1 lacking protein phosphatase 2C-A (PP2C-A) was similar to that in the WT, whereas little Ser1029 phosphorylation was observed in ABA-insensitive ark missense mutant lines. Furthermore, newly isolated ppabi1 ark lines showed ABA-insensitive phenotypes similar to those of ark lines. Therefore, ARK is a primary activator of SnRK2, preceding negative regulation by PP2C-A in bryophytes, which provides a prototype mechanism for ABA and abiotic stress responses in plants.
© American Society of Plant Biologists 2020. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Phosphoproteomic profiling reveals ABA-responsive phosphosignaling pathways in Physcomitrella patens.Plant J. 2018 May;94(4):699-708. doi: 10.1111/tpj.13891. Epub 2018 Apr 23. Plant J. 2018. PMID: 29575231
-
Sensor histidine kinases mediate ABA and osmostress signaling in the moss Physcomitrium patens.Curr Biol. 2022 Jan 10;32(1):164-175.e8. doi: 10.1016/j.cub.2021.10.068. Epub 2021 Nov 18. Curr Biol. 2022. PMID: 34798048
-
Plant Raf-like kinase integrates abscisic acid and hyperosmotic stress signaling upstream of SNF1-related protein kinase2.Proc Natl Acad Sci U S A. 2015 Nov 17;112(46):E6388-96. doi: 10.1073/pnas.1511238112. Epub 2015 Nov 4. Proc Natl Acad Sci U S A. 2015. PMID: 26540727 Free PMC article.
-
Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions.Genes Dev. 2010 Aug 15;24(16):1695-708. doi: 10.1101/gad.1953910. Genes Dev. 2010. PMID: 20713515 Free PMC article. Review.
-
Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants.Physiol Plant. 2013 Jan;147(1):15-27. doi: 10.1111/j.1399-3054.2012.01635.x. Epub 2012 May 16. Physiol Plant. 2013. PMID: 22519646 Review.
Cited by
-
Growth Promotion or Osmotic Stress Response: How SNF1-Related Protein Kinase 2 (SnRK2) Kinases Are Activated and Manage Intracellular Signaling in Plants.Plants (Basel). 2021 Jul 15;10(7):1443. doi: 10.3390/plants10071443. Plants (Basel). 2021. PMID: 34371646 Free PMC article. Review.
-
A framework for improving wheat spike development and yield based on the master regulatory TOR and SnRK gene systems.J Exp Bot. 2023 Feb 5;74(3):755-768. doi: 10.1093/jxb/erac469. J Exp Bot. 2023. PMID: 36477879 Free PMC article.
-
Unveiling the crucial roles of abscisic acid in plant physiology: implications for enhancing stress tolerance and productivity.Front Plant Sci. 2024 Nov 21;15:1437184. doi: 10.3389/fpls.2024.1437184. eCollection 2024. Front Plant Sci. 2024. PMID: 39640997 Free PMC article. Review.
-
Sucrose non-fermenting1-related protein kinase VcSnRK2.3 promotes anthocyanin biosynthesis in association with VcMYB1 in blueberry.Front Plant Sci. 2023 Feb 24;14:1018874. doi: 10.3389/fpls.2023.1018874. eCollection 2023. Front Plant Sci. 2023. PMID: 36909449 Free PMC article.
-
Plant hormone regulation of abiotic stress responses.Nat Rev Mol Cell Biol. 2022 Oct;23(10):680-694. doi: 10.1038/s41580-022-00479-6. Epub 2022 May 5. Nat Rev Mol Cell Biol. 2022. PMID: 35513717 Free PMC article. Review.
References
-
- Amagai A, Honda Y, Ishikawa S, Hara Y, Takezawa D, Sakata Y, Shinozaki K, Umezawa T (2018) Phosphoproteomic profiling reveals ABA-responsive phosphosignaling pathways in Physcomitrella patens. Plant J 94:699–708 - PubMed
-
- Bhyan SB, Minami A, Kaneko Y, Suzuki S, Arakawa K, Sakata Y, Takezawa D (2011) Cold acclimation in the moss Physcomitrella patens involves abscisic acid-dependent signaling. J Plant Physiol 169:137–145 - PubMed
-
- Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279:41758–41766 - PubMed
-
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Lauriere C (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63:491–503 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

