Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review)
- PMID: 33655327
- PMCID: PMC7834960
- DOI: 10.3892/ijmm.2021.4847
Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review)
Abstract
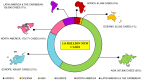
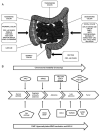
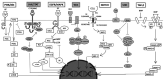
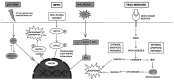
Colorectal cancer (CRC) is the third most frequently detected type of cancer, and the second most common cause of cancer‑related mortality globally. The American Cancer Society predicted that approximately 147,950 individuals would be diagnosed with CRC, out of which 53,200 individuals would succumb to the disease in the USA alone in 2020. CRC‑related mortality ranks third among both males and females in the USA. CRC arises from 3 major pathways: i) The adenoma‑carcinoma sequence; ii) serrated pathway; and iii) the inflammatory pathway. The majority of cases of CRC are sporadic and result from risk factors, such as a sedentary lifestyle, obesity, processed diets, alcohol consumption and smoking. CRC is also a common preventable cancer. With widespread CRC screening, the incidence and mortality from CRC have decreased in developed countries. However, over the past few decades, CRC cases and mortality have been on the rise in young adults (age, <50 years). In addition, CRC cases are increasing in developing countries with a low gross domestic product (GDP) due to lifestyle changes. CRC is an etiologically heterogeneous disease classified by tumor location and alterations in global gene expression. Accumulating genetic and epigenetic perturbations and aberrations over time in tumor suppressor genes, oncogenes and DNA mismatch repair genes could be a precursor to the onset of colorectal cancer. CRC can be divided as sporadic, familial, and inherited depending on the origin of the mutation. Germline mutations in APC and MLH1 have been proven to play an etiological role, resulting in the predisposition of individuals to CRC. Genetic alterations cause the dysregulation of signaling pathways leading to drug resistance, the inhibition of apoptosis and the induction of proliferation, invasion and migration, resulting in CRC development and metastasis. Timely detection and effective precision therapies based on the present knowledge of CRC is essential for successful treatment and patient survival. The present review presents the CRC incidence, risk factors, dysregulated signaling pathways and targeted therapies.
Keywords: colorectal cancer; signaling pathways; mutations; metastasis; targeted therapy.
Figures
Similar articles
-
Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies.Nat Rev Gastroenterol Hepatol. 2019 Dec;16(12):713-732. doi: 10.1038/s41575-019-0189-8. Epub 2019 Aug 27. Nat Rev Gastroenterol Hepatol. 2019. PMID: 31455888 Review.
-
Next-generation sequencing reveals heterogeneous genetic alterations in key signaling pathways of mismatch repair deficient colorectal carcinomas.Mod Pathol. 2020 Dec;33(12):2591-2601. doi: 10.1038/s41379-020-0612-2. Epub 2020 Jul 3. Mod Pathol. 2020. PMID: 32620917
-
A search for germline APC mutations in early onset colorectal cancer or familial colorectal cancer with normal DNA mismatch repair.Genes Chromosomes Cancer. 2001 Feb;30(2):181-6. Genes Chromosomes Cancer. 2001. PMID: 11135435
-
Germline Genetic Features of Young Individuals With Colorectal Cancer.Gastroenterology. 2018 Mar;154(4):897-905.e1. doi: 10.1053/j.gastro.2017.11.004. Epub 2017 Nov 14. Gastroenterology. 2018. PMID: 29146522 Free PMC article.
-
Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer.Int J Mol Sci. 2017 Jan 19;18(1):197. doi: 10.3390/ijms18010197. Int J Mol Sci. 2017. PMID: 28106826 Free PMC article. Review.
Cited by
-
Interferon-induced transmembrane protein 2 is a prognostic marker in colorectal cancer and promotes its progression by activating the PI3K/AKT pathway.Discov Oncol. 2024 May 27;15(1):191. doi: 10.1007/s12672-024-01040-x. Discov Oncol. 2024. PMID: 38802621 Free PMC article.
-
TSC22D2 Regulates ACOT8 to Delay the Malignant Progression of Colorectal Cancer.Onco Targets Ther. 2024 Mar 5;17:171-180. doi: 10.2147/OTT.S449244. eCollection 2024. Onco Targets Ther. 2024. PMID: 38476309 Free PMC article.
-
[HNRNPA1 gene is highly expressed in colorectal cancer: its prognostic implications and potential as a therapeutic target].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Sep 20;44(9):1685-1695. doi: 10.12122/j.issn.1673-4254.2024.09.08. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39505336 Free PMC article. Chinese.
-
Mindfulness intervention, homogeneous medical concept, and concentrated solution nursing for colorectal cancer patients: a retrospective study.BMC Cancer. 2024 Aug 27;24(1):1055. doi: 10.1186/s12885-024-12508-y. BMC Cancer. 2024. PMID: 39192195 Free PMC article.
-
A Benzimidazole-Based N-Heterocyclic Carbene Derivative Exhibits Potent Antiproliferative and Apoptotic Effects against Colorectal Cancer.Medicina (Kaunas). 2024 Aug 23;60(9):1379. doi: 10.3390/medicina60091379. Medicina (Kaunas). 2024. PMID: 39336420 Free PMC article.
References
-
- Graff RE, Möller S, Passarelli MN, Witte JS, Skytthe A, Christensen K, Tan Q, Adami HO, Czene K, Harris JR, et al. Familial risk and heritability of colorectal cancer in the nordic twin study of cancer. Clin Gastroenterol Hepatol. 2017;15:1256–1264. doi: 10.1016/j.cgh.2016.12.041. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

