Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer-an operational feasibility study
- PMID: 33655413
- PMCID: PMC7925259
- DOI: 10.1007/s00520-021-06099-8
Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer-an operational feasibility study
Abstract
Purpose: Pediatric patients with cancer are at high risk for severe infections. Infections can trigger changes of vital signs long before clinical symptoms arise. Continuous recording may detect such changes earlier than discrete measurements. We aimed to assess the feasibility of continuous recording of vital signs by a wearable device (WD) in pediatric patients undergoing chemotherapy for cancer.
Methods: In this prospective, observational single-center study, pediatric patients under chemotherapy wore the Everion® WD for 14 days. The predefined patient-specific goal was heart rate recorded in good quality during ≥18/24 h per day, on ≥7 consecutive days. The predefined criterion to claim feasibility was ≥15/20 patients fulfilling this patient-specific goal.
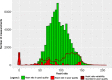
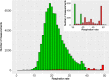
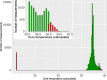
Results: Twenty patients were included (median age, 6 years; range, 2-16). Six patients aged 3-16 years fulfilled the patient-specific goal. Quality of heart rate recording was good during 3992 of 6576 (61%) hours studied and poor during 300 (5%) hours, and no data was recorded during 2284 (35%) hours. Eighteen of 20 participants indicated that this WD is acceptable to measure vital signs in children under chemotherapy.
Conclusion: The predefined feasibility criterion was not fulfilled. This was mainly due to important compliance problems and independent of the WD itself. However, continuous recording of vital signs was possible across a very wide age range in pediatric patients undergoing chemotherapy for cancer. We recommend to study feasibility in the Everion® again, plus in further WDs, applying measures to enhance compliance.
Trial registration: ClinicalTrials.gov (NCT04134429) on October 22, 2019.
Keywords: Continuous recording; Pediatric oncology; Recording vital signs; Supportive care; Wearable device.
© 2021. The Author(s).
Conflict of interest statement
Jochen Roessler reports personal fees from Pierre Fabre, Roche, Novartis, SOBI, Bayer, Jazz Pharmaceutical and Servier, all outside the submitted work. The other authors declare no competing interests.
Figures

References
-
- Lehrnbecher T, Robinson P, Fisher B, Alexander S, Ammann RA, Beauchemin M, Carlesse F, Groll AH, Haeusler GM, Santolaya M, Steinbach WJ, Castagnola E, Davis BL, Dupuis LL, Gaur AH, Tissing WJE, Zaoutis T, Phillips R, Sung L. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 Update. J Clin Oncol. 2017;35(18):2082–2094. doi: 10.1200/JCO.2016.71.7017. - DOI - PubMed
-
- Salstrom JL, Coughlin RL, Pool K, Bojan M, Mediavilla C, Schwent W, Rannie M, Law D, Finnerty M, Hilden J. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs. Pediatr Blood Cancer. 2015;62(5):807–815. doi: 10.1002/pbc.25435. - DOI - PMC - PubMed
-
- Koenig C, Schneider C, Morgan JE, Ammann RA, Sung L, Phillips B. Association of time to antibiotics and clinical outcomes in patients with fever and neutropenia during chemotherapy for cancer: a systematic review. Support Care Cancer. 2020;28(3):1369–1384. doi: 10.1007/s00520-019-04961-4. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

