Carbohydrate epitopes currently recognized as targets for IgE antibodies
- PMID: 33655520
- PMCID: PMC8489568
- DOI: 10.1111/all.14802
Carbohydrate epitopes currently recognized as targets for IgE antibodies
Abstract
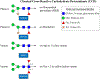
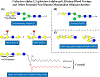
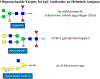
Until recently, glycan epitopes have not been documented by the WHO/IUIS Allergen Nomenclature Sub-Committee. This was in part due to scarce or incomplete information on these oligosaccharides, but also due to the widely held opinion that IgE to these epitopes had little or no relevance to allergic symptoms. Most IgE-binding glycans recognized up to 2008 were considered to be "classical" cross-reactive carbohydrate determinants (CCD) that occur in insects, some helminths and throughout the plant kingdom. Since 2008, the prevailing opinion on lack of clinical relevance of IgE-binding glycans has been subject to a reevaluation. This was because IgE specific for the mammalian disaccharide galactose-alpha-1,3-galactose (alpha-gal) was identified as a cause of delayed anaphylaxis to mammalian meat in the United States, an observation that has been confirmed by allergists in many parts of the world. Several experimental studies have shown that oligosaccharides with one or more terminal alpha-gal epitopes can be attached as a hapten to many different mammalian proteins or lipids. The classical CCDs also behave like haptens since they can be expressed on proteins from multiple species. This is the explanation for extensive in vitro cross-reactivity related to CCDs. Because of these developments, the Allergen Nomenclature Sub-Committee recently decided to include glycans as potentially allergenic epitopes in an adjunct section of its website (www.allergen.org). In this article, the features of the main glycan groups known to be involved in IgE recognition are revisited, and their characteristic structural, functional, and clinical features are discussed.
Keywords: MMXF; MUXF3; O-glycans; alpha-gal; clinical relevance; cross-reactive carbohydrate determinants; hapten.
© 2021 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Figures


References
-
- Aalberse RC, Koshte V, Clemens JGJ. Immunoglobulin-E antibodies that crossreact with vegetable foods, pollen, and hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356–364. - PubMed
-
- Altmann F The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142(2):99–115. - PubMed
-
- van Ree R Clinical importance of cross-reactivity in food allergy. Curr Opin Allergy Clin Immunol. 2004;4(3):235–240. - PubMed
-
- Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol. 2017;140(2):356–368. - PubMed
-
- Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130(1):155–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

