Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release
- PMID: 33656514
- PMCID: PMC7933984
- DOI: 10.1084/jem.20201452
Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release
Abstract
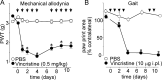
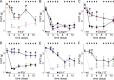
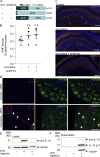
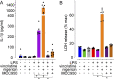
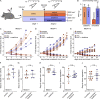
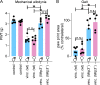
Vincristine is an important component of many regimens used for pediatric and adult malignancies, but it causes a dose-limiting sensorimotor neuropathy for which there is no effective treatment. This study aimed to delineate the neuro-inflammatory mechanisms contributing to the development of mechanical allodynia and gait disturbances in a murine model of vincristine-induced neuropathy, as well as to identify novel treatment approaches. Here, we show that vincristine-induced peripheral neuropathy is driven by activation of the NLRP3 inflammasome and subsequent release of interleukin-1β from macrophages, with mechanical allodynia and gait disturbances significantly reduced in knockout mice lacking NLRP3 signaling pathway components, or after treatment with the NLRP3 inhibitor MCC950. Moreover, treatment with the IL-1 receptor antagonist anakinra prevented the development of vincristine-induced neuropathy without adversely affecting chemotherapy efficacy or tumor progression in patient-derived medulloblastoma xenograph models. These results detail the neuro-inflammatory mechanisms leading to vincristine-induced peripheral neuropathy and suggest that repurposing anakinra may be an effective co-treatment strategy to prevent vincristine-induced peripheral neuropathy.
© 2021 Starobova et al.
Conflict of interest statement
Disclosures: K. Schroder reported "other" from Inflazome Ltd outside the submitted work; in addition, K. Schroder had a patent to PCT/EP2017/053498 licensed (Inflazome Ltd), a patent to PCT/IB2017/053059 licensed (Inflazome Ltd), and a patent to PCT/AU2016/050103 licensed (Inflazome Ltd); served on the Scientific Advisory Board of Inflazome in 2016-2017; and serves as a consultant to Quench Bio, USA and Novartis, Switzerland. No other disclosures were reported.
Figures

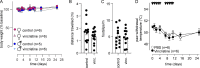






Comment in
-
Immune-mediated vincristine-induced neuropathy: Unlocking therapies.J Exp Med. 2021 May 3;218(5):e20210286. doi: 10.1084/jem.20210286. J Exp Med. 2021. PMID: 33751022 Free PMC article.
Similar articles
-
NET-Triggered NLRP3 Activation and IL18 Release Drive Oxaliplatin-Induced Peripheral Neuropathy.Cancer Immunol Res. 2022 Dec 2;10(12):1542-1558. doi: 10.1158/2326-6066.CIR-22-0197. Cancer Immunol Res. 2022. PMID: 36255412 Free PMC article.
-
Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model.J Neuroinflammation. 2019 Apr 10;16(1):78. doi: 10.1186/s12974-019-1459-7. J Neuroinflammation. 2019. PMID: 30971286 Free PMC article.
-
Role of the NLRP3 inflammasome in a model of acute burn-induced pain.Burns. 2017 Mar;43(2):304-309. doi: 10.1016/j.burns.2016.09.001. Epub 2016 Dec 28. Burns. 2017. PMID: 28040362
-
MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation.J Neuroinflammation. 2020 Aug 31;17(1):256. doi: 10.1186/s12974-020-01933-y. J Neuroinflammation. 2020. PMID: 32867797 Free PMC article.
-
Unlocking New Therapeutic Options for Vincristine-Induced Neuropathic Pain: The Impact of Preclinical Research.Life (Basel). 2024 Nov 17;14(11):1500. doi: 10.3390/life14111500. Life (Basel). 2024. PMID: 39598298 Free PMC article. Review.
Cited by
-
NET-Triggered NLRP3 Activation and IL18 Release Drive Oxaliplatin-Induced Peripheral Neuropathy.Cancer Immunol Res. 2022 Dec 2;10(12):1542-1558. doi: 10.1158/2326-6066.CIR-22-0197. Cancer Immunol Res. 2022. PMID: 36255412 Free PMC article.
-
Inhibition of the NLRP3 inflammasome using MCC950 reduces vincristine-induced adverse effects in an acute lymphoblastic leukemia patient-derived xenograft model.Hemasphere. 2025 Mar 18;9(3):e70092. doi: 10.1002/hem3.70092. eCollection 2025 Mar. Hemasphere. 2025. PMID: 40104043 Free PMC article.
-
Macrophage depletion blocks congenital SARM1-dependent neuropathy.J Clin Invest. 2022 Dec 1;132(23):e159800. doi: 10.1172/JCI159800. J Clin Invest. 2022. PMID: 36287209 Free PMC article.
-
MCC950 Reduces the Anxiodepressive-like Behaviors and Memory Deficits Related to Paclitaxel-Induced Peripheral Neuropathy in Mice.Antioxidants (Basel). 2025 Jan 25;14(2):143. doi: 10.3390/antiox14020143. Antioxidants (Basel). 2025. PMID: 40002330 Free PMC article.
-
Neuroprotective Effect of Polyvalent Immunoglobulins on Mouse Models of Chemotherapy-Induced Peripheral Neuropathy.Pharmaceutics. 2024 Jan 20;16(1):139. doi: 10.3390/pharmaceutics16010139. Pharmaceutics. 2024. PMID: 38276509 Free PMC article.
References
-
- Abbate, A., Salloum F.N., Vecile E., Das A., Hoke N.N., Straino S., Biondi-Zoccai G.G., Houser J.E., Qureshi I.Z., Ownby E.D., et al. . 2008. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 117:2670–2683. 10.1161/CIRCULATIONAHA.107.740233 - DOI - PubMed
-
- Amaya, F., Wang H., Costigan M., Allchorne A.J., Hatcher J.P., Egerton J., Stean T., Morisset V., Grose D., Gunthorpe M.J., et al. . 2006. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 26:12852–12860. 10.1523/JNEUROSCI.4015-06.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

