Phenotypic switch of smooth muscle cells in paediatric chronic intestinal pseudo-obstruction syndrome
- PMID: 33656779
- PMCID: PMC8051695
- DOI: 10.1111/jcmm.16367
Phenotypic switch of smooth muscle cells in paediatric chronic intestinal pseudo-obstruction syndrome
Abstract
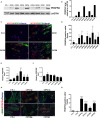
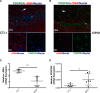
Smooth Muscle Cells (SMC) are unique amongst all muscle cells in their capacity to modulate their phenotype. Indeed, SMCs do not terminally differentiate but instead harbour a remarkable capacity to dedifferentiate, switching between a quiescent contractile state and a highly proliferative and migratory phenotype, a quality often associated to SMC dysfunction. However, phenotypic plasticity remains poorly examined in the field of gastroenterology in particular in pathologies in which gut motor activity is impaired. Here, we assessed SMC status in biopsies of infants with chronic intestinal pseudo-obstruction (CIPO) syndrome, a life-threatening intestinal motility disorder. We showed that CIPO-SMCs harbour a decreased level of contractile markers. This phenotype is accompanied by an increase in Platelet-Derived Growth Factor Receptor-alpha (PDGFRA) expression. We showed that this modulation occurs without origin-related differences in CIPO circular and longitudinal-derived SMCs. As we characterized PDGFRA as a marker of digestive mesenchymal progenitors during embryogenesis, our results suggest a phenotypic switch of the CIPO-SMC towards an undifferentiated stage. The development of CIPO-SMC culture and the characterization of SMC phenotypic switch should enable us to design therapeutic approaches to promote SMC differentiation in CIPO.
Keywords: PDGFR pathway; chronic intestinal pseudo-obstruction Disease; intestinal motility disorders; plasticity; smooth muscle cells.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures




References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767‐801. - PubMed
-
- Scirocco A, Matarrese P, Carabotti M, et al. Cellular and Molecular Mechanisms of Phenotypic Switch in Gastrointestinal Smooth Muscle. J. Cell. Physiol. 2016;231:295‐302. - PubMed
-
- Knowles CH, De Giorgio R, Kapur RP, et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59:882‐887. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

