Multigene PCR using both cfDNA and cfRNA in the supernatant of pleural effusion achieves accurate and rapid detection of mutations and fusions of driver genes in patients with advanced NSCLC
- PMID: 33656807
- PMCID: PMC7982639
- DOI: 10.1002/cam4.3769
Multigene PCR using both cfDNA and cfRNA in the supernatant of pleural effusion achieves accurate and rapid detection of mutations and fusions of driver genes in patients with advanced NSCLC
Abstract
Background: Pleural effusion from patients with advanced non-small cell lung cancer (NSCLC) has been proved valuable for molecular analysis, especially when the tissue sample not available. However, simultaneous detection of multiple driver gene alterations especially the fusions is still challenging.
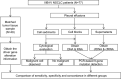
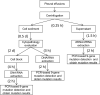
Methods: In this study, 77 patients with advanced NSCLC and pleural effusion were enrolled, 49 of whom had matched tumor tissues. Supernatants, cell sediments, and cell blocks were prepared from pleural effusion samples for detection of driver alterations by a PCR-based 9-gene mutation detection kit.
Results: Mutations in EGFR, KRAS, and HER2 were detected in DNA and cfDNA, fusions in ALK was detected in RNA and cfRNA. Compared with matched tumor tissue, the supernatant showed the highest overall sensitivity (81.3%), with 81.5% for SNV/Indels by cfDNA and 80% for fusions by cfRNA, followed by cell blocks (71.0%) and the cell sediments (66.7%). Within the group of treatment-naïve patients or malignant cells observed in the cell sediments, supernatant showed higher overall sensitivity (89.5% and 92.3%) with both 100% for fusions.
Conclusions: CfDNA and cfRNA derived from pleural effusion supernatant have been successfully tested with a PCR-based multigene detection kit. Pleural effusion supernatant seems a preferred material for detection of multigene alterations to guide treatment decision of advanced NSCLC.
Keywords: ALK fusion; NSCLC; PCR; cfRNA; pleural effusion; supernatant.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Guanshan Zhu is the stock holder of Amoy Diagnostics.
Figures
References
-
- Zhou C, Wu Y‐L, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735‐742. 10.1016/s1470-2045(11)70184-x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

