Analysis of the reporting of adverse drug reactions in children and adolescents in Germany in the time period from 2000 to 2019
- PMID: 33657139
- PMCID: PMC7928460
- DOI: 10.1371/journal.pone.0247446
Analysis of the reporting of adverse drug reactions in children and adolescents in Germany in the time period from 2000 to 2019
Abstract
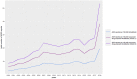
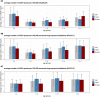
The objective of this study was to analyse reports on adverse drug reactions (ADRs) from Germany in the particularly vulnerable patient group of children and adolescents. Reporting characteristics, demographic parameters and off-label use were examined among others. The ratio of ADR reports per number of German inhabitants and the ratio of ADR reports per number of German inhabitants exposed to drugs were calculated and compared. These parameters were examined to derive trends in reporting of ADRs. 20,854 spontaneous ADR reports for the age group 0-17 years were identified in the European ADR database EudraVigilance for the time period 01.01.2000-28.02.2019 and analysed with regard to the aforementioned criteria. 86.5% (18,036/20,854) of the ADR reports originated from Healthcare Professionals and 12.2% (2,546/20,854) from non-Healthcare Professionals. 74.4% (15,522/20,854) of the ADR reports were classified as serious. The proportion of ADR reports per age group was 11.8% (0-1 month), 11.0% (2 months-1 year), 7.4% (2-3 years), 9.3% (4-6 years), 25.8% (7-12 years), and 34.8% (13-17 years) years, respectively. Male sex slightly dominated (51.2% vs. 44.8% females). Only 3.5% of the ADR reports reported off-label use. The annual number of ADR reports increased since 2000, even if set in context with the number of inhabitants and assumed drug-exposed inhabitants. The pediatric population declined in the study period which argues against its prominent role for the increase in the total number of ADR reports. Instead, among others, changes in reporting obligations may apply. The high proportion of serious ADR reports underlines the importance of pediatric drug safety.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. British Journal of Clinical Pharmacology. 2001;52:77–83. 10.1046/j.0306-5251.2001.01407.x - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

