Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation
- PMID: 33657225
- PMCID: PMC8493975
- DOI: 10.1182/blood.2020009396
Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation
Abstract
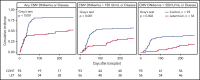
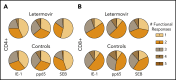
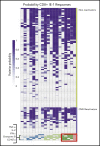
Decreased cytomegalovirus (CMV)-specific immunity after hematopoietic cell transplantation (HCT) is associated with late CMV reactivation and increased mortality. Whether letermovir prophylaxis-associated reduction in viral exposure influences CMV-specific immune reconstitution is unknown. In a prospective cohort of allogeneic HCT recipients who received letermovir, we compared polyfunctional CMV-specific T-cell responses to those of controls who received PCR-guided preemptive therapy before the introduction of letermovir. Thirteen-color flow cytometry was used to assess T-cell responses at 3 months after HCT following stimulation with CMV immediate early-1 (IE-1) antigen and phosphoprotein 65 (pp65) antigens. Polyfunctionality was characterized by combinatorial polyfunctionality analysis of antigen-specific T-cell subsets. Use of letermovir and reduction of viral exposure were assessed for their association with CMV-specific T-cell immunity. Polyfunctional T-cell responses to IE-1 and pp65 were decreased in letermovir recipients and remained diminished after adjustment for donor CMV serostatus, absolute lymphocyte count, and steroid use. Among letermovir recipients, greater peak CMV DNAemia and increased viral shedding were associated with stronger CD8+ responses to pp65, whereas the CMV shedding rate was associated with greater CD4+ responses to IE-1. In summary, our study provided initial evidence that letermovir may delay CMV-specific cellular reconstitution, possibly related to decreased CMV antigen exposure. Evaluating T-cell polyfunctionality may identify patients at risk for late CMV infection after HCT.
© 2021 by The American Society of Hematology.
Figures





Comment in
-
Letermovir and HCT: too much of a good thing?Blood. 2021 Jul 8;138(1):1-2. doi: 10.1182/blood.2021011459. Blood. 2021. PMID: 34236425 Free PMC article.
-
Letermovir prophylaxis in T-cell-depleted transplants: breakthrough and rebound infections in the postmarketing setting.Blood Adv. 2021 Nov 9;5(21):4500-4503. doi: 10.1182/bloodadvances.2021005637. Blood Adv. 2021. PMID: 34614515 Free PMC article. No abstract available.
References
-
- Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylasix for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444. - PubMed
-
- Marty FM, Ljungman PT, Chemaly RF, et al. Outcomes of patients with detectable CMV DNA at randomization in the phase III trial of letermovir for the prevention of CMV infection in allogeneic hematopoietic cell transplantation. Am J Transplant. 2020;20(6):1703-1711. - PubMed
-
- Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407-414. - PubMed
-
- Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060-3067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

