Drivers of dynamic intratumor heterogeneity and phenotypic plasticity
- PMID: 33657326
- PMCID: PMC8163571
- DOI: 10.1152/ajpcell.00575.2020
Drivers of dynamic intratumor heterogeneity and phenotypic plasticity
Abstract
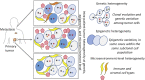
Cancer is a clonal disease, i.e., all tumor cells within a malignant lesion trace their lineage back to a precursor somatic cell that acquired oncogenic mutations during development and aging. And yet, those tumor cells tend to have genetic and nongenetic variations among themselves-which is denoted as intratumor heterogeneity. Although some of these variations are inconsequential, others tend to contribute to cell state transition and phenotypic heterogeneity, providing a substrate for somatic evolution. Tumor cell phenotypes can dynamically change under the influence of genetic mutations, epigenetic modifications, and microenvironmental contexts. Although epigenetic and microenvironmental changes are adaptive, genetic mutations are usually considered permanent. Emerging reports suggest that certain classes of genetic alterations show extensive reversibility in tumors in clinically relevant timescales, contributing as major drivers of dynamic intratumor heterogeneity and phenotypic plasticity. Dynamic heterogeneity and phenotypic plasticity can confer resistance to treatment, promote metastasis, and enhance evolvability in cancer. Here, we first highlight recent efforts to characterize intratumor heterogeneity at genetic, epigenetic, and microenvironmental levels. We then discuss phenotypic plasticity and cell state transition by tumor cells, under the influence of genetic and nongenetic determinants and their clinical significance in classification of tumors and therapeutic decision-making.
Keywords: cancer evolution; cell state transition; drug resistance; intratumor heterogeneity; phenotypic plasticity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Maley CC, Aktipis A, Graham TA, Sottoriva A, Boddy AM, Janiszewska M, Silva AS, Gerlinger M, Yuan Y, Pienta KJ, Anderson KS, Gatenby R, Swanton C, Posada D, Wu CI, Schiffman JD, Hwang ES, Polyak K, Anderson ARA, Brown JS, Greaves M, Shibata D. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer 17: 605–619, 2017. doi: 10.1038/nrc.2017.69. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

