Avoid the trap: Targeting PARP1 beyond human malignancy
- PMID: 33657415
- PMCID: PMC8052287
- DOI: 10.1016/j.chembiol.2021.02.004
Avoid the trap: Targeting PARP1 beyond human malignancy
Abstract
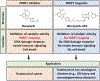
PARP1 is a poly(ADP-ribose) polymerase (PARP) enzyme that plays a critical role in regulating DNA damage response. The main enzymatic function of PARP1 is to catalyze a protein post-translational modification known as poly(ADP-ribosyl)ation (PARylation). Human cancers with homologous recombination deficiency are highly sensitive to PARP1 inhibitors. PARP1 is aberrantly activated in many non-oncological diseases, leading to the excessive NAD+ depletion and PAR formation, thus causing cell death and tissue damage. PARP1 deletion offers a profound protective effect in the relevant animal models. However, many of the current PARP1 inhibitors also induce PARP1 trapping, which drives subsequent DNA damage, innate immune response and cytotoxicity. This minireview provides an overview of the basic biology of PARP1 trapping, and its implications in disease. Furthermore, we also discuss the recent development of PARP1 PROTAC compounds, and their utility as "non-trapping" PARP1 degraders for the potential amelioration of non-oncological diseases driven by aberrant PARP1 activation.
Keywords: BRCA; NAD(+); PARP; breast cancer; cell death; ischemia reperfusion injury; neurodegeneration; ovarian cancer; phase transition; poly(ADP-ribose); stroke.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests A provisional patent application on the PARP degraders and technologies described herein was previously filed (Y.Y. and C.C.).
Figures
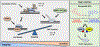
Similar articles
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
PARP1: A Promising Target for the Development of PARP1-based Candidates for Anticancer Intervention.Curr Med Chem. 2016;23(17):1756-74. doi: 10.2174/0929867321666140915143516. Curr Med Chem. 2016. PMID: 25245372 Review.
-
Aminomethylmorpholino Nucleosides as Novel Inhibitors of PARP1 and PARP2: Experimental and Molecular Modeling Analyses of Their Selectivity and Mechanism of Action.Int J Mol Sci. 2024 Nov 22;25(23):12526. doi: 10.3390/ijms252312526. Int J Mol Sci. 2024. PMID: 39684238 Free PMC article.
-
High affinity and low PARP-trapping benzimidazole derivatives as a potential warhead for PARP1 degraders.Eur J Med Chem. 2024 May 5;271:116405. doi: 10.1016/j.ejmech.2024.116405. Epub 2024 Apr 25. Eur J Med Chem. 2024. PMID: 38678823
-
Medicinal chemistry approaches of poly ADP-Ribose polymerase 1 (PARP1) inhibitors as anticancer agents - A recent update.Eur J Med Chem. 2019 Mar 1;165:198-215. doi: 10.1016/j.ejmech.2019.01.024. Epub 2019 Jan 12. Eur J Med Chem. 2019. PMID: 30684797 Review.
Cited by
-
The role of E3 ubiquitin ligase WWP2 and the regulation of PARP1 by ubiquitinated degradation in acute lymphoblastic leukemia.Cell Death Discov. 2022 Oct 18;8(1):421. doi: 10.1038/s41420-022-01209-9. Cell Death Discov. 2022. PMID: 36257929 Free PMC article.
-
Therapeutic Potentials of Poly (ADP-Ribose) Polymerase 1 (PARP1) Inhibition in Multiple Sclerosis and Animal Models: Concept Revisiting.Adv Sci (Weinh). 2022 Feb;9(5):e2102853. doi: 10.1002/advs.202102853. Epub 2021 Dec 21. Adv Sci (Weinh). 2022. PMID: 34935305 Free PMC article. Review.
-
Effects of food and ethnicity on the pharmacokinetics of venadaparib, a next-generation PARP inhibitor, in healthy Korean, Caucasian, and Chinese male subjects.Invest New Drugs. 2024 Feb;42(1):80-88. doi: 10.1007/s10637-023-01405-z. Epub 2023 Dec 15. Invest New Drugs. 2024. PMID: 38099989 Free PMC article. Clinical Trial.
-
Replication stress as a driver of cellular senescence and aging.Commun Biol. 2024 May 22;7(1):616. doi: 10.1038/s42003-024-06263-w. Commun Biol. 2024. PMID: 38777831 Free PMC article. Review.
-
PINX1 loss confers susceptibility to PARP inhibition in pan-cancer cells.Cell Death Dis. 2024 Aug 22;15(8):610. doi: 10.1038/s41419-024-07009-6. Cell Death Dis. 2024. PMID: 39174499 Free PMC article.
References
-
- Banasik M, Komura H, Shimoyama M, and Ueda K (1992). Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferase. The Journal of biological chemistry 267, 1569–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

