Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies
- PMID: 33657424
- PMCID: PMC7904446
- DOI: 10.1016/j.chom.2021.02.019
Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies
Abstract
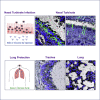
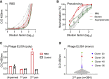
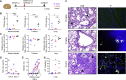
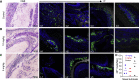
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is characterized by a burst in the upper respiratory portal for high transmissibility. To determine human neutralizing antibodies (HuNAbs) for entry protection, we tested three potent HuNAbs (IC50 range, 0.0007-0.35 μg/mL) against live SARS-CoV-2 infection in the golden Syrian hamster model. These HuNAbs inhibit SARS-CoV-2 infection by competing with human angiotensin converting enzyme-2 for binding to the viral receptor binding domain (RBD). Prophylactic intraperitoneal or intranasal injection of individual HuNAb or DNA vaccination significantly reduces infection in the lungs but not in the nasal turbinates of hamsters intranasally challenged with SARS-CoV-2. Although postchallenge HuNAb therapy suppresses viral loads and lung damage, robust infection is observed in nasal turbinates treated within 1-3 days. Our findings demonstrate that systemic HuNAb suppresses SARS-CoV-2 replication and injury in lungs; however, robust viral infection in nasal turbinate may outcompete the antibody with significant implications to subprotection, reinfection, and vaccine.
Keywords: COVID-19; SARS-CoV-2; human neutralizing antibody; lung injury; nasal turbinate; phage display; receptor binding domain; upper respiratory tract.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.F.-W.C. has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited and was an invited speaker for Gilead Sciences Hong Kong Limited and Luminex Corporation. The other authors declare no conflicts of interest except for a provisional patent application filed for human monoclonal antibodies generated in our laboratory by the University of Hong Kong. The funding sources had no role in study design, data collection, analysis, interpretation, or writing of the report.
Figures

Similar articles
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Sex Differences in Lung Imaging and SARS-CoV-2 Antibody Responses in a COVID-19 Golden Syrian Hamster Model.mBio. 2021 Aug 31;12(4):e0097421. doi: 10.1128/mBio.00974-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253053 Free PMC article.
-
Neutralizing Monoclonal Antibodies That Target the Spike Receptor Binding Domain Confer Fc Receptor-Independent Protection against SARS-CoV-2 Infection in Syrian Hamsters.mBio. 2021 Oct 26;12(5):e0239521. doi: 10.1128/mBio.02395-21. Epub 2021 Sep 14. mBio. 2021. PMID: 34517754 Free PMC article.
-
Antibodies, epicenter of SARS-CoV-2 immunology.Cell Death Differ. 2021 Feb;28(2):821-824. doi: 10.1038/s41418-020-00711-w. Epub 2021 Jan 26. Cell Death Differ. 2021. PMID: 33500559 Free PMC article. Review. No abstract available.
-
Human neutralizing antibodies for SARS-CoV-2 prevention and immunotherapy.Immunother Adv. 2021 Dec 30;2(1):ltab027. doi: 10.1093/immadv/ltab027. eCollection 2022. Immunother Adv. 2021. PMID: 35915816 Free PMC article. Review.
Cited by
-
In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains.Res Sq [Preprint]. 2021 Apr 23:rs.3.rs-448370. doi: 10.21203/rs.3.rs-448370/v1. Res Sq. 2021. Update in: Nature. 2021 Aug;596(7870):103-108. doi: 10.1038/s41586-021-03720-y. PMID: 34013259 Free PMC article. Updated. Preprint.
-
COVID-19 Animal Models and Vaccines: Current Landscape and Future Prospects.Vaccines (Basel). 2021 Sep 26;9(10):1082. doi: 10.3390/vaccines9101082. Vaccines (Basel). 2021. PMID: 34696190 Free PMC article. Review.
-
Antiviral Drugs and Vaccines for Omicron Variant: A Focused Review.Can J Infect Dis Med Microbiol. 2023 Sep 8;2023:6695533. doi: 10.1155/2023/6695533. eCollection 2023. Can J Infect Dis Med Microbiol. 2023. PMID: 37719798 Free PMC article. Review.
-
Generation and Effect Testing of a SARS-CoV-2 RBD-Targeted Polyclonal Therapeutic Antibody Based on a 2-D Airway Organoid Screening System.Front Immunol. 2021 Oct 18;12:689065. doi: 10.3389/fimmu.2021.689065. eCollection 2021. Front Immunol. 2021. PMID: 34733269 Free PMC article.
-
Developing Recombinant Antibodies by Phage Display Against Infectious Diseases and Toxins for Diagnostics and Therapy.Front Cell Infect Microbiol. 2021 Jul 7;11:697876. doi: 10.3389/fcimb.2021.697876. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34307196 Free PMC article. Review.
References
-
- Chames J.M.P. In: Antibody Engineering: Methods and Protocols. Second Edition. Chames P., editor. Humana Press Inc.; 2012. Phage display and selections on purified antigens; pp. 213–224. - PubMed
-
- Chan J.F.-W., Zhang A.J., Yuan S., Poon V.K.-M., Chan C.C.-S., Lee A.C.-Y., Chan W.-M., Fan Z., Tsoi H.-W., Wen L., et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases. 2020;71:2428–2446. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

