Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization
- PMID: 33657435
- PMCID: PMC8989071
- DOI: 10.1016/j.neubiorev.2021.02.040
Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization
Abstract
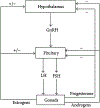
Brain development is a life-long process that encompasses several critical periods of transition, during which significant cognitive changes occur. Embryonic development, puberty, and reproductive senescence are all periods of transition that are hypersensitive to environmental factors. Rather than isolated episodes, each transition builds upon the last and is influenced by consequential changes that occur in the transition before it. Epigenetic marks, such as DNA methylation and histone modifications, provide mechanisms by which early events can influence development, cognition, and health outcomes. For example, parental environment influences imprinting patterns in gamete cells, which ultimately impacts gene expression in the embryo which may result in hypersensitivity to poor maternal nutrition during pregnancy, raising the risks for cognitive impairment later in life. This review explores how epigenetics induce and regulate critical periods, and also discusses how early environmental interactions prime a system towards a particular health outcome and influence susceptibility to disease or cognitive impairment throughout life.
Keywords: Aging; Andropause; B-vitamins; DNA methylation; Epigenetics; Estrogen; Histone modification; Menopause; Neurodegeneration; Neurodevelopmental; One-carbon metabolism; Perimenopause; Puberty.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
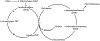

References
-
- Abdolmaleky HM, Zhou JR, Thiagalingam S, 2015. An update on the epigenetics of psychotic diseases and autism. Epigenomics 7 (3), 427–449. - PubMed
-
- Adleman NE, et al. , 2002. A developmental fMRI study of the Stroop color-word task. Neuroimage 16 (1), 61–75. - PubMed
-
- Albright CD, et al. , 1999a. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res 113 (1–2), 13–20. - PubMed
-
- Albright CD, et al. , 1999b. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. Dev. Brain Res 115 (2), 123–129. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

