Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada
- PMID: 33657636
- PMCID: PMC9643049
- DOI: 10.1055/s-0041-1725146
Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada
Abstract
Objective: This study aimed to evaluate palivizumab (PVZ) use, trends in indications, and outcomes of respiratory illness hospitalizations (RIH) and respiratory syncytial virus hospitalizations (RSVH).
Study design: It involves a large, Canadian prospective (2005-2017) observational multicenter study of children at high risk for RSV infection.
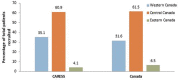
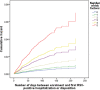
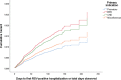
Results: A total of 25,003 infants (56.3% male) were enrolled at 32 sites; 109,579 PVZ injections were administered. Indications included: prematurity (63.3%); "miscellaneous" (17.8%); hemodynamically significant congenital heart disease (10.5%); bronchopulmonary dysplasia/chronic lung disease (8.4%). The "miscellaneous" group increased over time (4.4% in 2005-2006 to 22.5% in 2016-2017) and included: trisomy 21, airway anomalies, pulmonary disorders, cystic fibrosis, neurological impairments, immunocompromised, cardiac aged >2 years, multiple conditions, and a residual "unclassified" group. Adherence measured by expected versus actual doses plus correct interdose interval was 64.7%. A total of 2,054 RIH occurred (6.9%); 198 (9.6%) required intubation. Three hundred thirty-seven hospitalized children were RSV-positive (overall RSVH 1.6%). Risk factors for RSVH included having siblings, attending daycare, family history of atopy, smoking exposure, and crowded household. Infants with 5 risk factors were 9.0 times (95% CI or confidence interval 4.4-18.2; p < 0.0005) more likely to have RSVH than infants without risk factors. Three adverse events occurred; none were fatal.
Conclusion: Results are relevant to both clinicians and decision-makers. We confirmed the safety of PVZ. Use of PVZ increased steadily for children with miscellaneous conditions and medical complexity. Medical and social factors pose a risk for severe RIH and RSVH with accompanying burden of illness. A vaccine that protects against RSV is urgently required.
Key points: · Main indications were prematurity (63.3%); "miscellaneous" (17.8%); hemodynamically significant congenital heart disease (10.5%); bronchopulmonary dysplasia/chronic lung disease (8.4%).. · The proportion of children in the "miscellaneous" group, comprised of those with trisomy 21, airway anomalies, pulmonary disorders, cystic fibrosis, neurological impairments, immunocompromised, cardiac aged >2 years, multiple conditions, and a residual "unclassified" group, increased over time (4.4% in 2005-2006 to 22.5% in 2016-2017).. · Respiratory illness-related hospitalization occurred in 2,054 children (6.9%); 198 (9.6%) required intubation. Three hundred thirty-seven hospitalized children were RSV-positive (overall RSVH: 1.6%)..
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
B.A.P., I.M., K.L.L., and C.L.B. have received investigator-initiated research funding or received compensation as advisors or lecturers from AbbVie Corporation, Merck and MedImmune. A.L. has no conflicts of interest to disclose. I.M. reports grant from AbbVie CANADA, other from AbbVie Canada, during the conduct of the study; grants from Regeneron, grants from Medimmune, outside the submitted work. C.L.B. reports grant from Abbvie, during the conduct of the study; other from Regeneron, outside the submitted work. B.A.P. reports grants and personal fees from AbbVie Incorporated, personal fees from Merck, during the conduct of the study. K.L.L. reports grants and personal fees from AbbVie, outside the submitted work. A.L. reports grant from AbbVie Canada, during the conduct of the study.
Figures
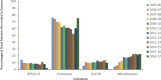
References
-
- Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development—a global agenda. Vaccine. 2016;34(26):2870–2875. - PubMed
-
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group Pediatrics 1998102(3, pt. 1):531–537. - PubMed
-
- Cardiac Synagis Study Group . Feltes T F, Cabalka A K, Meissner H C. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(04):532–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

