Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation
- PMID: 33657648
- PMCID: PMC8013891
- DOI: 10.1111/all.14794
Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation
Abstract
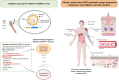
Following the emergency use authorization of the mRNA-1273 vaccine on the 18th of December 2020, two mRNA vaccines are in current use for the prevention of coronavirus disease 2019 (COVID-19). For both mRNA vaccines, the phase III pivotal trials excluded individuals with a history of allergy to vaccine components. Immediately after the initiation of vaccination in the United Kingdom, Canada, and the United States, anaphylactic reactions were reported. While the culprit trigger requires investigation, initial reports suggested the excipient polyethylene glycol 2000 (PEG-2000)-contained in both vaccines as the PEG-micellar carrier system-as the potential culprit. Surface PEG chains form a hydrate shell to increase stability and prevent opsonization. Allergic reactions to such PEGylated lipids can be IgE-mediated, but may also result from complement activation-related pseudoallergy (CARPA) that has been described in similar liposomes. In addition, mRNA-1273 also contains tromethamine (trometamol), which has been reported to cause anaphylaxis to substances such as gadolinium-based contrast media. Skin prick, intradermal and epicutaneous tests, in vitro sIgE assessment, evaluation of sIgG/IgM, and basophil activation tests are being used to demonstrate allergic reactions to various components of the vaccines.
Keywords: COVID; SARS-CoV; vaccines.
© 2021 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Conflict of interest statement
Dr. Akdis reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne—Center for Allergy Research and Education, European Commission's Horison's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Scibase, advisory role in Sanofi/Regeneron, grants from Glakso Smith‐Kline, advisory role in Scibase. Dr. Bousquet reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi‐Aventis, Takeda, Teva, Uriach, other from KYomed Innov, outside the submitted work. Dr. Jutel reports personal fees from ALK‐Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, Leti, Biomay, HAL, during the conduct of the study; Astra‐Zeneka, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, Medimmune, Chiesi, outside the submitted work. Dr. Klimek reports grants and/or personal fees from Allergopharma, MEDA/Mylan, HAL Allergie, ALK‐Abelló, from LETI Pharma, Stallergenes, Quintiles, Sanofi, ASIT biotech, Lofarma, Allergy Therapeut., AstraZeneca, GSK, Inmunotk, Cassella med, outside the submitted work; and reports Membership: AeDA, DGHNO, Deutsche Akademie für Allergologie und klinischeImmunologie, HNO‐BV, GPA, EAACI. Dr. Novak reports grants and/or personal fees from ALK‐Abello, HAL Allergy, Stallergenes Geer, Leti Pharma, Novartis, Leo Pharma, Abbvie, Sanofi Genzmye, Lofarma, Bencard Allergy Therapeutics, outside the submitted work. Dr. Cabanillas has nothing to disclose.
Figures

References
-
- FDA . United States Food and Drug Administration (FDA). Emergency use authorization for Moderna COVIC‐19 vaccine. https://www.FDA.gov/media/144636/download. Accessed December 28, 2020.
-
- FDA . United States Food and Drug Administration (FDA). Emergency use authorization for Pfizer‐BioNTech COVID‑19 Vaccine. https://www.fda.gov/media/144412/download. Accessed December 28, 2020.
-
- Moderna I. Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID‐19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization. 30 November 2020. Cambridge, UK: Moderna, Inc.; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

